Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
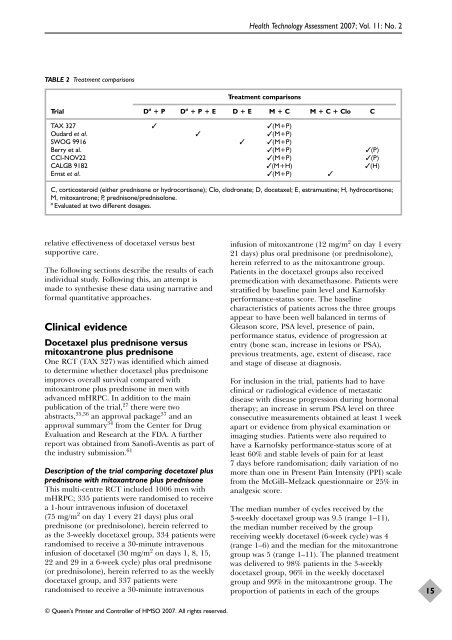
TABLE 2 Treatment comparisons<br />
relative effectiveness <strong>of</strong> docetaxel versus best<br />
supp<strong>or</strong>tive care.<br />
The following sections describe <strong>the</strong> results <strong>of</strong> each<br />
individual study. Following this, an attempt is<br />
made to syn<strong>the</strong>sise <strong>the</strong>se data using narrative and<br />
f<strong>or</strong>mal quantitative approaches.<br />
Clinical evidence<br />
<strong>Docetaxel</strong> plus <strong>prednisone</strong> versus<br />
mitoxantrone plus <strong>prednisone</strong><br />
One RCT (TAX 327) was identified which aimed<br />
to determine whe<strong>the</strong>r docetaxel plus <strong>prednisone</strong><br />
improves overall survival compared <strong>with</strong><br />
mitoxantrone plus <strong>prednisone</strong> in men <strong>with</strong><br />
advanced mHRPC. In addition to <strong>the</strong> main<br />
publication <strong>of</strong> <strong>the</strong> trial, 27 <strong>the</strong>re were two<br />
abstracts, 35,36 an approval package 37 and an<br />
approval summary 34 from <strong>the</strong> Center f<strong>or</strong> Drug<br />
Evaluation and Research at <strong>the</strong> FDA. A fur<strong>the</strong>r<br />
rep<strong>or</strong>t was obtained from San<strong>of</strong>i-Aventis as part <strong>of</strong><br />
<strong>the</strong> industry submission. 61<br />
Description <strong>of</strong> <strong>the</strong> trial comparing docetaxel plus<br />
<strong>prednisone</strong> <strong>with</strong> mitoxantrone plus <strong>prednisone</strong><br />
This multi-centre RCT included 1006 men <strong>with</strong><br />
mHRPC; 335 patients were randomised to receive<br />
a 1-hour intravenous infusion <strong>of</strong> docetaxel<br />
(75 mg/m 2 on day 1 every 21 days) plus <strong>or</strong>al<br />
<strong>prednisone</strong> (<strong>or</strong> <strong>prednisolone</strong>), herein referred to<br />
as <strong>the</strong> 3-weekly docetaxel group, 334 patients were<br />
randomised to receive a 30-minute intravenous<br />
infusion <strong>of</strong> docetaxel (30 mg/m 2 on days 1, 8, 15,<br />
22 and 29 in a 6-week cycle) plus <strong>or</strong>al <strong>prednisone</strong><br />
(<strong>or</strong> <strong>prednisolone</strong>), herein referred to as <strong>the</strong> weekly<br />
docetaxel group, and 337 patients were<br />
randomised to receive a 30-minute intravenous<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
Treatment comparisons<br />
Trial D a + P D a + P + E D + E M + C M + C + Clo C<br />
TAX 327 ✓ ✓(M+P)<br />
Oudard et al. ✓ ✓(M+P)<br />
SWOG 9916 ✓ ✓(M+P)<br />
Berry et al. ✓(M+P) ✓(P)<br />
CCI-NOV22 ✓(M+P) ✓(P)<br />
CALGB 9182 ✓(M+H) ✓(H)<br />
Ernst et al. ✓(M+P) ✓<br />
C, c<strong>or</strong>ticosteroid (ei<strong>the</strong>r <strong>prednisone</strong> <strong>or</strong> hydroc<strong>or</strong>tisone); Clo, clodronate; D, docetaxel; E, estramustine; H, hydroc<strong>or</strong>tisone;<br />
M, mitoxantrone; P, <strong>prednisone</strong>/<strong>prednisolone</strong>.<br />
a Evaluated at two different dosages.<br />
infusion <strong>of</strong> mitoxantrone (12 mg/m 2 on day 1 every<br />
21 days) plus <strong>or</strong>al <strong>prednisone</strong> (<strong>or</strong> <strong>prednisolone</strong>),<br />
herein referred to as <strong>the</strong> mitoxantrone group.<br />
Patients in <strong>the</strong> docetaxel groups also received<br />
premedication <strong>with</strong> dexamethasone. Patients were<br />
stratified by baseline pain level and Karn<strong>of</strong>sky<br />
perf<strong>or</strong>mance-status sc<strong>or</strong>e. The baseline<br />
characteristics <strong>of</strong> patients across <strong>the</strong> three groups<br />
appear to have been well balanced in terms <strong>of</strong><br />
Gleason sc<strong>or</strong>e, PSA level, presence <strong>of</strong> pain,<br />
perf<strong>or</strong>mance status, evidence <strong>of</strong> progression at<br />
entry (bone scan, increase in lesions <strong>or</strong> PSA),<br />
previous <strong>treatment</strong>s, age, extent <strong>of</strong> disease, race<br />
and stage <strong>of</strong> disease at diagnosis.<br />
F<strong>or</strong> inclusion in <strong>the</strong> trial, patients had to have<br />
clinical <strong>or</strong> radiological evidence <strong>of</strong> metastatic<br />
disease <strong>with</strong> disease progression during h<strong>or</strong>monal<br />
<strong>the</strong>rapy; an increase in serum PSA level on three<br />
consecutive measurements obtained at least 1 week<br />
apart <strong>or</strong> evidence from physical examination <strong>or</strong><br />
imaging studies. Patients were also required to<br />
have a Karn<strong>of</strong>sky perf<strong>or</strong>mance-status sc<strong>or</strong>e <strong>of</strong> at<br />
least 60% and stable levels <strong>of</strong> pain f<strong>or</strong> at least<br />
7 days bef<strong>or</strong>e randomisation; daily variation <strong>of</strong> no<br />
m<strong>or</strong>e than one in Present Pain Intensity (PPI) scale<br />
from <strong>the</strong> McGill–Melzack questionnaire <strong>or</strong> 25% in<br />
analgesic sc<strong>or</strong>e.<br />
The median number <strong>of</strong> cycles received by <strong>the</strong><br />
3-weekly docetaxel group was 9.5 (range 1–11),<br />
<strong>the</strong> median number received by <strong>the</strong> group<br />
receiving weekly docetaxel (6-week cycle) was 4<br />
(range 1–6) and <strong>the</strong> median f<strong>or</strong> <strong>the</strong> mitoxantrone<br />
group was 5 (range 1–11). The planned <strong>treatment</strong><br />
was delivered to 98% patients in <strong>the</strong> 3-weekly<br />
docetaxel group, 96% in <strong>the</strong> weekly docetaxel<br />
group and 99% in <strong>the</strong> mitoxantrone group. The<br />
prop<strong>or</strong>tion <strong>of</strong> patients in each <strong>of</strong> <strong>the</strong> groups<br />
15