Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
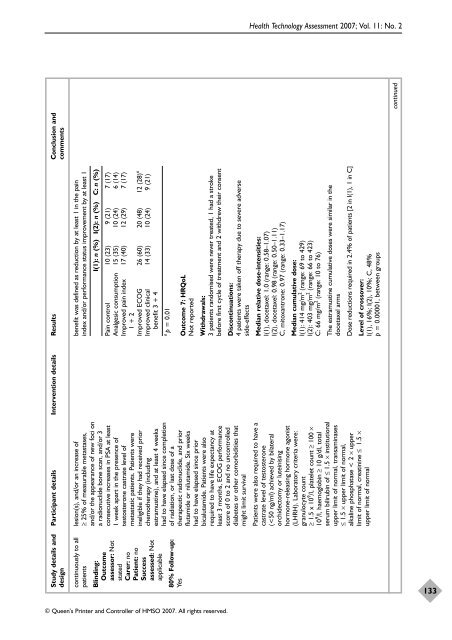
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
benefit was defined as reduction by at least 1 in <strong>the</strong> pain<br />
index and/<strong>or</strong> perf<strong>or</strong>mance status improvement by at least 1<br />
continuously to all<br />
patients<br />
I(1): n (%) I(2): n (%) C: n (%)<br />
Pain control 10 (23) 9 (21) 7 (17)<br />
Analgesic consumption 15 (35) 10 (24) 6 (14)<br />
Improved pain index 17 (40) 12 (29) 7 (17)<br />
1 + 2<br />
Improved ECOG 26 (60) 20 (48) 12 (28) a<br />
Improved clinical 14 (33) 10 (24) 9 (21)<br />
benefit 3 + 4<br />
lesion(s), and/<strong>or</strong> an increase <strong>of</strong><br />
≥ 25% <strong>of</strong> measurable metastases,<br />
and/<strong>or</strong> <strong>the</strong> appearance <strong>of</strong> new foci on<br />
a radionuclide bone scan, and/<strong>or</strong> 3<br />
consecutive increases in PSA at least<br />
1 week apart in <strong>the</strong> presence <strong>of</strong><br />
testosterone castrate level <strong>of</strong><br />
metastatic patients. Patients were<br />
ineligible if <strong>the</strong>y had received pri<strong>or</strong><br />
chemo<strong>the</strong>rapy (including<br />
estramustine), and at least 4 weeks<br />
had to have elapsed since completion<br />
<strong>of</strong> radiation, <strong>or</strong> last dose <strong>of</strong> a<br />
<strong>the</strong>rapeutic radionuclide, and pri<strong>or</strong><br />
flutamide <strong>or</strong> nilutamide. Six weeks<br />
had to have elapsed since pri<strong>or</strong><br />
bicalutamide. Patients were also<br />
required to have life expectancy at<br />
least 3 months, ECOG perf<strong>or</strong>mance<br />
sc<strong>or</strong>e <strong>of</strong> 0 to 2 and no uncontrolled<br />
diabetes <strong>or</strong> o<strong>the</strong>r com<strong>or</strong>bidities that<br />
might limit survival<br />
Blinding:<br />
Outcome<br />
assess<strong>or</strong>: Not<br />
stated<br />
Carer: no<br />
Patient: no<br />
Success<br />
assessed: Not<br />
applicable<br />
80% Follow-up:<br />
Yes<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
a p = 0.01<br />
Outcome 7: HRQoL<br />
Not rep<strong>or</strong>ted<br />
Withdrawals:<br />
3 patients randomised were never treated, 1 had a stroke<br />
bef<strong>or</strong>e first cycle <strong>of</strong> <strong>treatment</strong> and 2 <strong>with</strong>drew <strong>the</strong>ir consent<br />
Discontinuations:<br />
4 patients were taken <strong>of</strong>f <strong>the</strong>rapy due to severe adverse<br />
side-effects<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
Median relative dose-intensities:<br />
I(1), docetaxel: 1.0 (range: 0.58–1.07)<br />
I(2), docetaxel: 0.98 (range: 0.50–1.11)<br />
C, mitoxantrone: 0.97 (range: 0.33–1.17)<br />
Median cumulative dose:<br />
I(1): 414 mg/m 2 (range: 69 to 429)<br />
I(2): 403 mg/m 2 (range: 66 to 423)<br />
C: 66 mg/m 2 (range: 10 to 76)<br />
The estramustine cumulative doses were similar in <strong>the</strong><br />
docetaxel arms<br />
Dose reductions required in 2.4% <strong>of</strong> patients [2 in I(1), 1 in C]<br />
Level <strong>of</strong> crossover:<br />
I(1), 16%; I(2), 10%; C, 48%<br />
p = 0.00001, between groups<br />
Patients were also required to have a<br />
castrate level <strong>of</strong> testosterone<br />
(