Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
136<br />
Appendix 6<br />
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
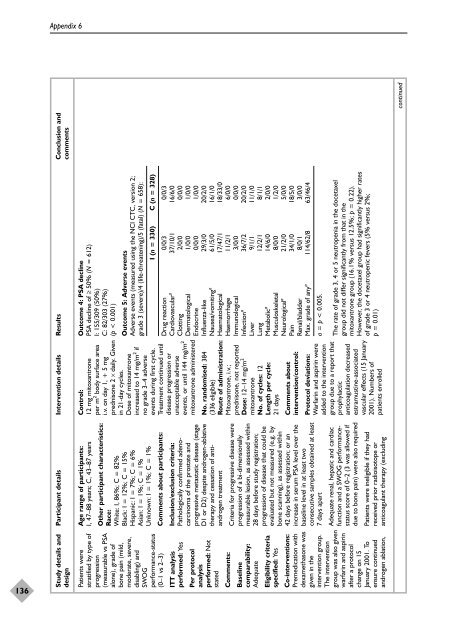
Outcome 4: PSA decline<br />
PSA decline <strong>of</strong> ≥ 50% (N = 612)<br />
I: 155/309 (50%)<br />
C: 82/303 (27%)<br />
(p < 0.001)<br />
Outcome 5: Adverse events<br />
Adverse events (measured using <strong>the</strong> NCI CTC, version 2;<br />
grade 3 (severe)/4 (life-threatening)/5 (fatal) (N = 658):<br />
I (n = 330) C (n = 328)<br />
Control:<br />
12 mg mitoxantrone<br />
per m 2 body surface area<br />
i.v. on day 1, + 5 mg<br />
<strong>prednisone</strong> 2 × daily. Given<br />
in 21-day cycles.<br />
Dose <strong>of</strong> mitoxantrone<br />
increased to 14 mg/m 2 if<br />
no grade 3–4 adverse<br />
events during first cycle.<br />
Treatment continued until<br />
disease progression <strong>or</strong><br />
unacceptable adverse<br />
events, <strong>or</strong> until 144 mg/m 2<br />
mitoxantrone administered<br />
Age range <strong>of</strong> participants:<br />
I, 47–88 years; C, 43–87 years<br />
O<strong>the</strong>r participant characteristics:<br />
Race:<br />
White: I, 86%; C = 82%<br />
Black: I = 12%; C = 15%<br />
Hispanic: I = 7%; C = 6%<br />
Asian: I = 1%; C = 1%<br />
Unknown: I = 1%; C = 1%<br />
Comments about participants:<br />
Patients were<br />
stratified by type <strong>of</strong><br />
progression<br />
(measurable vs PSA<br />
alone), grade <strong>of</strong><br />
bone pain (mild,<br />
moderate, severe,<br />
disabling) and<br />
SWOG<br />
perf<strong>or</strong>mance-status<br />
(0–1 vs 2–3)<br />
Drug reaction 0/0/3 0/0/3<br />
Cardiovascular a<br />
37/10/1 16/6/0<br />
Clotting 2/0/0 0/0/0<br />
Dermatological 1/0/0 1/0/0<br />
Endocrine 0/0/0 1/0/0<br />
Influenza-like 29/3/0 20/2/0<br />
Nausea/vomiting a<br />
61/5/0 16/1/0<br />
Haematological 17/47/1 18/33/0<br />
Haem<strong>or</strong>rhage 11/2/1 6/0/0<br />
Immunological 3/0/0 0/0/0<br />
Infection a<br />
36/7/2 20/2/0<br />
Liver 9/1/1 11/1/0<br />
Lung 12/2/1 8/1/1<br />
Metabolic a<br />
14/6/0 2/0/0<br />
Musculoskeletal 8/0/0 1/2/0<br />
Neurological a<br />
21/2/0 5/0/0<br />
Pain 34/1/0 18/5/0<br />
Renal/bladder 8/0/1 3/0/0<br />
Max. grade <strong>of</strong> any a<br />
114/62/8 63/46/4<br />
Inclusion/exclusion criteria:<br />
Pathologically confirmed adenocarcinoma<br />
<strong>of</strong> <strong>the</strong> prostate and<br />
progressive metastatic disease (stage<br />
D1 <strong>or</strong> D2) despite androgen-ablative<br />
<strong>the</strong>rapy and cessation <strong>of</strong> antiandrogen<br />
<strong>treatment</strong><br />
ITT analysis<br />
perf<strong>or</strong>med: Yes<br />
No. randomised: 384<br />
(336 eligible)<br />
Route <strong>of</strong> administration:<br />
Mitoxantrone, i.v.;<br />
<strong>prednisone</strong>, not rep<strong>or</strong>ted<br />
Dose: 12–14 mg/m 2<br />
mitoxantrone<br />
No. <strong>of</strong> cycles: 12<br />
Length per cycle:<br />
21 days<br />
Per protocol<br />
analysis<br />
perf<strong>or</strong>med: Not<br />
stated<br />
Criteria f<strong>or</strong> progressive disease were<br />
progression <strong>of</strong> a bi-dimensionally<br />
measurable lesion, as assessed <strong>with</strong>in<br />
28 days bef<strong>or</strong>e study registration;<br />
progression <strong>of</strong> disease that could be<br />
evaluated but not measured (e.g. by<br />
bone scanning), as assessed <strong>with</strong>in<br />
42 days bef<strong>or</strong>e registration; <strong>or</strong> an<br />
increase in serum PSA level over <strong>the</strong><br />
baseline level in at least two<br />
consecutive samples obtained at least<br />
7 days apart<br />
Comments:<br />
Baseline<br />
comparability:<br />
Adequate<br />
Comments about<br />
intervention/control:<br />
a = p < 0.005.<br />
The rate <strong>of</strong> grade 3, 4 <strong>or</strong> 5 neutropenia in <strong>the</strong> docetaxel<br />
group did not differ significantly from that in <strong>the</strong><br />
mitoxantrone group (16.1% versus 12.5%; p = 0.22).<br />
However, <strong>the</strong> docetaxel group had significantly higher rates<br />
<strong>of</strong> grade 3 <strong>or</strong> 4 neutropenic fevers (5% versus 2%;<br />
p = 0.01)<br />
Protocol deviations:<br />
Warfarin and aspirin were<br />
added to <strong>the</strong> intervention<br />
group due to a rep<strong>or</strong>t that<br />
prophylactic<br />
anticoagulation decreased<br />
estramustine-associated<br />
vascular effects (15 January<br />
2001). Numbers <strong>of</strong><br />
patients enrolled<br />
Adequate renal, hepatic and cardiac<br />
function and a SWOG perf<strong>or</strong>mancestatus<br />
sc<strong>or</strong>e <strong>of</strong> 0–2 (3 was allowed if<br />
due to bone pain) were also required<br />
Patients were ineligible if <strong>the</strong>y had<br />
received pri<strong>or</strong> radioisotope <strong>or</strong><br />
anticoagulant <strong>the</strong>rapy (excluding<br />
Eligibility criteria<br />
specified: Yes<br />
Co-interventions:<br />
Premedication <strong>with</strong><br />
dexamethasone was<br />
given in <strong>the</strong><br />
intervention group.<br />
The intervention<br />
group was also given<br />
warfarin and aspirin<br />
after a protocol<br />
change on 15<br />
January 2001. To<br />
ensure continued<br />
androgen ablation,<br />
continued