Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Docetaxel with prednisone or prednisolone for the treatment of ...
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Study details and Participant details Intervention details Results Conclusion and<br />
design comments<br />
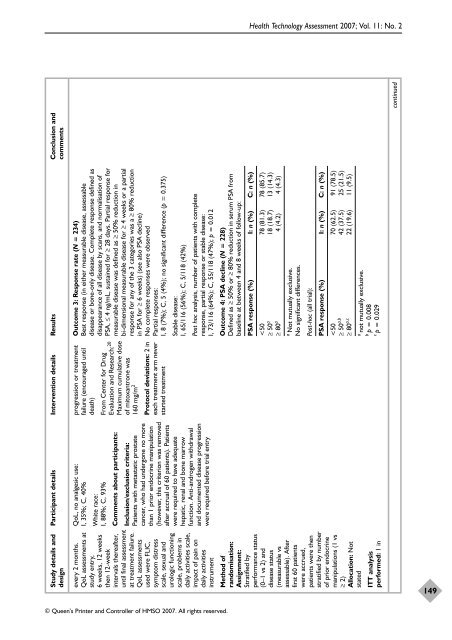
Outcome 3: Response rate (N = 234)<br />
Best response (in ei<strong>the</strong>r measurable disease, assessable<br />
disease <strong>or</strong> bone-only disease. Complete response defined as<br />
disappearance <strong>of</strong> all disease by scans, and n<strong>or</strong>malisation <strong>of</strong><br />
PSA, ≤ 4 ng/mL, sustained f<strong>or</strong> ≥ 28 days. Partial response f<strong>or</strong><br />
measurable disease was defined as ≥ 50% reduction in<br />
bi-dimensional measurable disease f<strong>or</strong> ≥ 4 weeks <strong>or</strong> a partial<br />
response f<strong>or</strong> any <strong>of</strong> <strong>the</strong> 3 categ<strong>or</strong>ies was a ≥ 80% reduction<br />
in PSA f<strong>or</strong> ≥ 6 weeks) (see also PSA decline)<br />
No complete responses were observed<br />
Partial responses:<br />
I, 8 (7%); C, 5 (4%); no significant difference (p = 0.375)<br />
progression <strong>or</strong> <strong>treatment</strong><br />
failure (encouraged until<br />
death)<br />
QoL, no analgesic use:<br />
I, 35%; C, 40%<br />
From Center f<strong>or</strong> Drug<br />
Evaluation and Research: 20<br />
Maximum cumulative dose<br />
<strong>of</strong> mitoxantrone was<br />
160 mg/m 2<br />
Protocol deviations: 2 in<br />
each <strong>treatment</strong> arm never<br />
started <strong>treatment</strong><br />
Stable disease:<br />
I, 65/116 (56%); C, 5/118 (42%)<br />
Post hoc analysis, number <strong>of</strong> patients <strong>with</strong> complete<br />
response, partial response <strong>or</strong> stable disease:<br />
I, 73/116 (64%); C, 55/118 (47%); p = 0.012<br />
White race:<br />
I, 88%; C, 93%<br />
Comments about participants:<br />
Inclusion/exclusion criteria:<br />
Patients <strong>with</strong> metastatic prostate<br />
cancer, who had undergone no m<strong>or</strong>e<br />
than 1 pri<strong>or</strong> endocrine manipulation<br />
(however, this criterion was removed<br />
after accrual <strong>of</strong> 60 patients). Patients<br />
were required to have adequate<br />
hepatic, renal and bone marrow<br />
function. Anti-androgen <strong>with</strong>drawal<br />
and documented disease progression<br />
were required bef<strong>or</strong>e trial entry<br />
every 2 months.<br />
QoL assessments at<br />
study entry,<br />
6 weeks, 12 weeks<br />
<strong>the</strong>n 12-week<br />
intervals <strong>the</strong>reafter,<br />
until final assessment<br />
at <strong>treatment</strong> failure.<br />
QoL assessments<br />
used were FLIC,<br />
symptom distress<br />
scale, sexual and<br />
urologic functioning<br />
scale, problems in<br />
daily activities scale,<br />
impact <strong>of</strong> pain on<br />
daily activities<br />
instrument<br />
© Queen’s Printer and Controller <strong>of</strong> HMSO 2007. All rights reserved.<br />
Outcome 4: PSA decline (N = 228)<br />
Defined as ≥ 50% <strong>or</strong> ≥ 80% reduction in serum PSA from<br />
baseline at between 4 and 8 weeks <strong>of</strong> follow-up:<br />
Health Technology Assessment 2007; Vol. 11: No. 2<br />
PSA response (%) I: n (%) C: n (%)<br />