- Page 1 and 2:
Annual Pro
- Page 3:
-i- INTRODUCTION The March 2002 <st
- Page 6 and 7:
Barley Transformation G.J. Muehlbau
- Page 9 and 10:
1 2000/2001 WINTER NURSERY: BARLEY
- Page 11 and 12:
3 2001 Barley Stripe Rust Screening
- Page 13 and 14:
5 Triumph/Tyra//Arupo, a selection
- Page 15 and 16:
7 Washington (Fossum Cereals and Wa
- Page 17 and 18:
9 This project is supported by the
- Page 19 and 20:
11 ‘Triumph’, ‘Valier’, ‘
- Page 21 and 22:
13 Table 2. Agronomic data for sele
- Page 23 and 24:
15 Table 4. Agronomic data for sele
- Page 25 and 26:
17 Table 8. Malting quality data* f
- Page 27 and 28:
19 Six-rowed barley selections of c
- Page 29 and 30:
21 Table 14. Agronomic data for sel
- Page 31 and 32:
23 Table 16. Agronomic data for win
- Page 33 and 34:
25 Table 19. Malting quality data*
- Page 35 and 36:
Garnet/98Ab11865 Garnet/98Ab12895 9
- Page 37 and 38:
29 rust resistant NSGC accessions o
- Page 39 and 40:
31 DEVELOPMENT OF SIX-ROWED MALTING
- Page 41 and 42:
33 backcrossing. The selfed lines w
- Page 43 and 44:
35 MINNESOTA BARLEY IMPROVEMENT PRO
- Page 45 and 46:
37 ADVANCED LINE EVALUATION Varieti
- Page 47 and 48:
39 OTHER BARLEY DISEASES In 2001, r
- Page 49 and 50:
41 Wingbermuehle, W. J., K.M. Belin
- Page 51 and 52:
43 In 2001 winter/spring, over 300
- Page 53 and 54:
45 assessments were made by countin
- Page 55 and 56:
47 IDENTIFICATION OF NOVEL DISEASE
- Page 57 and 58:
49 Unfortunately, disease levels in
- Page 59 and 60:
51 BARLEY TRANSFORMATION G.J. Muehl
- Page 61 and 62:
53 We have available the sugarcane
- Page 63 and 64:
55 Secondly, we tried to grow F. gr
- Page 65 and 66:
57 References: Issac, S. and Jennin
- Page 67 and 68:
59 Huntley-dryland) with two replic
- Page 69 and 70:
61 Table 2. 1992 thru 2001 Overall
- Page 71 and 72:
63 performance for Montana farmers.
- Page 73 and 74:
65 Blake, T,. Hensleigh P., Boss D.
- Page 75 and 76:
67 settling tower cannot accommodat
- Page 77 and 78:
Table 3. Seedling reaction to strip
- Page 79 and 80:
71 TWO-ROWED BARLEY IMPROVEMENT PRO
- Page 81 and 82:
Table 1. Agronomic trait comparison
- Page 83 and 84:
75 mutants reduce plant vigor and g
- Page 85 and 86:
77 SIX-ROWED BARLEY IMPROVEMENT PRO
- Page 87 and 88:
79 this variety has decreased over
- Page 89 and 90:
Table 7. Malt quality comparisons o
- Page 91 and 92:
83 • Management studies for malt
- Page 93 and 94:
85 STUDIES ON BARLEY DISEASES AND T
- Page 95 and 96:
87 With funding from the US Wheat a
- Page 97 and 98:
89 MALTING AND BREWING QUALITY OF B
- Page 99 and 100:
91 β-glucans, it might be most ins
- Page 101 and 102:
10 9 8 7 6 5 4 3 2 1 0 93 Figure 1.
- Page 103 and 104:
95 Bolin, P., Schwarz, P., Jones, B
- Page 105 and 106:
97 Results Results from field and g
- Page 107 and 108:
99 DEVELOPING BARLEY TISSUE CULTURE
- Page 109 and 110:
101 trials of plants regenerated fr
- Page 111 and 112: 103 Table 2. Agronomic performance
- Page 113 and 114: 105 BSMV spread between entries. Ho
- Page 115 and 116: 107 involving two susceptible backg
- Page 117 and 118: 109 THE OREGON BARLEY IMPROVEMENT P
- Page 119 and 120: 111 for traits that are difficult/e
- Page 121 and 122: Germplasm Development: 113 Crosses:
- Page 123 and 124: 115 • All lines in spring yield t
- Page 125 and 126: 117 Table 2. Agronomic data for Ore
- Page 127 and 128: 119 Table 6. Malting quality of win
- Page 129 and 130: 121 Table 7. Across location summar
- Page 131 and 132: 123 MOLECULAR MARKER ASSISTED MODIF
- Page 133 and 134: 125
- Page 135 and 136: 127 exchanges. Spring and winter ba
- Page 137 and 138: 129 Table 2. 2000-2001 Agronomic Pe
- Page 139 and 140: 131 Table 7. Spring 2- and 6-Row #
- Page 141 and 142: 133 2. Direct Seeding Cropping Syst
- Page 143 and 144: 135 D.M. Wesenberg - spring and win
- Page 145 and 146: 137 A STUDY OF THE MALTING QUALITY
- Page 147 and 148: 139 Methods. Our malting schedule.
- Page 149 and 150: 141 modification measures indicated
- Page 151 and 152: 143 CHARACTERIZATION OF THE PROTEIN
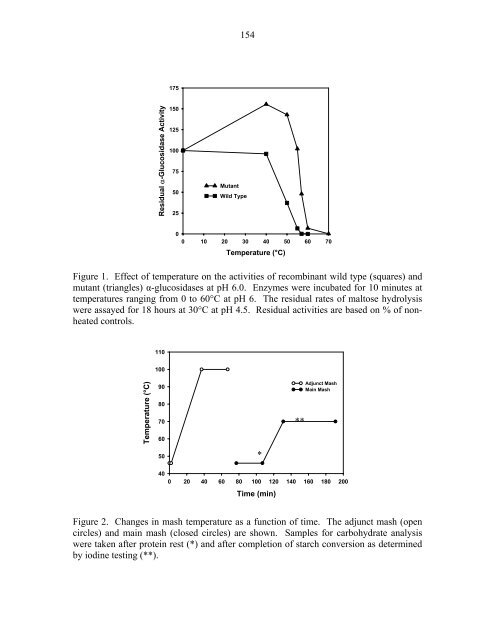
- Page 153 and 154: 145 Purification of a serine endope
- Page 155 and 156: 147 STUDIES ON THE PROTEINASES THAT
- Page 157 and 158: 149 with the proteinase T will be s
- Page 159 and 160: 151 10°C increase in thermostabili
- Page 161: 153 Sissons MJ and MacGregor AW (19
- Page 165 and 166: 157 Tissue-specific gene products f
- Page 167 and 168: 159 The promoters were subcloned fr
- Page 169: 161 Fig. 3. Expression of Trx-Hth f