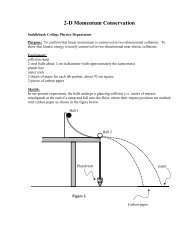
Saddleback Journal of Biology - Saddleback College
Saddleback Journal of Biology - Saddleback College
Saddleback Journal of Biology - Saddleback College
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Fall 2009 <strong>Biology</strong> 3B Paper<br />
The peak pH <strong>of</strong> bicarbonate-stimulated saliva was<br />
greater than that produced by chewing the standard<br />
gum at all times. It represents a decrease in the<br />
hydrogen ion concentration <strong>of</strong> the whole mouth saliva,<br />
thus yields greater pH value. The salivary bicarbonate<br />
concentration is known to increase with increasing<br />
flow rate (Dawes 1969). The bicarbonate concentration<br />
<strong>of</strong> gum-stimulated saliva has been reported to increase<br />
from an unstimulated value around 4mM to a peak <strong>of</strong><br />
15mM when chewing a 3 gram gum sample<br />
(Rosenhack, et al, 1993). It is because the rise in<br />
salivary pH there was an increase in salivary<br />
bicarbonate concentrations. The bicarbonate gum<br />
pellets contain 3 percent sodium bicarbonate, and it is<br />
most likely that the additional increase in salivary pH<br />
with the bicarbonate gum was due to bicarbonate ions<br />
leaching out from the gum. As this reservoir diminishes<br />
with time, the differences in pH <strong>of</strong> the saliva stimulated<br />
by each gum will decreases. The rate at which gum<br />
ingredients enter the saliva has been estimated by<br />
measuring the salivary sucrose levels in participants<br />
chewing sucrose-containing gum (Rosenhack, et.al.,<br />
1993). It was found that most <strong>of</strong> the sucrose was lost<br />
over 10-15minutes, depending on the size <strong>of</strong> the<br />
sample (Rosenhack, et.al., 1993). These data are not<br />
consistent with the time course <strong>of</strong> the salivary pH<br />
changes induced by chewing bicarbonate gum in the<br />
present experiment (Fig.1). This study presents new<br />
findings that the bicarbonate chewing gum delayed the<br />
loss <strong>of</strong> bicarbonate by approximately 10 minutes, over<br />
20-25 minute interval. The higher salivary pH achieved<br />
with chewing bicarbonate gum, compared to a<br />
standard, sugar-free gum, may have important oral<br />
health implications.<br />
Acknowledgements<br />
The authors wish to thank Pr<strong>of</strong>essor Steve Teh <strong>of</strong> the<br />
Biological Sciences Department, <strong>Saddleback</strong> <strong>College</strong><br />
for his technical assistance and devotion to the success<br />
<strong>of</strong> this investigation. Also thank all the volunteers who<br />
donated their precious saliva. Special thanks goes to<br />
Dr. Tran, D.D.S. for his suggestion on conducting this<br />
investigation.<br />
Literature Cited<br />
Dawes, C. and Macpherson L.M. (1992). Effects <strong>of</strong><br />
nine different chewing-gums and lozenges on salivary<br />
flow rate and pH. Caries Res. 26:176-182.<br />
Dawes, C. (1969). The effects <strong>of</strong> flow rate and duration<br />
<strong>of</strong> stimulation on the concentrations <strong>of</strong> protein and the<br />
main electrolytes in human parotid saliva. Archives <strong>of</strong><br />
Oral <strong>Biology</strong>.14:277-280.<br />
Dawes, C and Puckett, D.C. (1995). The effects <strong>of</strong><br />
chewing frequency and duration <strong>of</strong> gum chewing on<br />
salivary flow rate and sucrose concentrations. Arch<br />
Oral Biol. 40:585-588.<br />
Dawes, C. (1997). Effects <strong>of</strong> a bicarbonate-containing<br />
dentrifrice on pH changes in a gel-stabilized plaque<br />
after exposure to sucrose. Compendium Continuin<br />
Educ Dentistry Suppl. 18(21): 8-10.<br />
Dawes, C. (2003). What is the critical pH and why<br />
does a tooth dissolve in acid?. J Can Dent Assoc.<br />
69(11):722-724.<br />
DePaola, D.P. (2008). Saliva: The precious body fluid.<br />
J Am Dent Assoc. 139:5S-10S.<br />
Gracia-Godoy, F. and Hicks, J.M. (2008).Maintaining<br />
the integrity <strong>of</strong> the enamel surface: The role <strong>of</strong> dental<br />
bi<strong>of</strong>ilm, saliva and preventive agents in enamel<br />
demineralization and remineralization. J Am Dent<br />
Assoc. 139:25-34.<br />
Legier-Vargas,K. (1995). Effects <strong>of</strong> sodium<br />
bicarbonate dentifrices on the levels <strong>of</strong> cariogenic<br />
bacteria in human saliva. Caries Res. 29: 143-147.<br />
Rosenhek, M., Macpherson, L.M., and Dawes, C.<br />
(1993). The effects <strong>of</strong> chewing-gum stick size and<br />
duration <strong>of</strong> chewing on salivary flow rate and sucrosebicarbonate<br />
concentrations. Arch Oral Bio. 38-885-<br />
891.<br />
119<br />
<strong>Saddleback</strong> <strong>Journal</strong> <strong>of</strong> <strong>Biology</strong><br />
Spring 2010