Saddleback Journal of Biology - Saddleback College
Saddleback Journal of Biology - Saddleback College
Saddleback Journal of Biology - Saddleback College
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Fall 2009 <strong>Biology</strong> 3B Paper<br />
environments to determine if urban run<strong>of</strong>f can<br />
significantly increase the degree <strong>of</strong> eutrophication. We<br />
hypothesized that rural lakes would have a lower<br />
concentration <strong>of</strong> soluble phosphorous than lakes in<br />
urban environments.<br />
Methods and Materials<br />
Ten 3.5 L water samples were taken from<br />
lakes in California. Five <strong>of</strong> the lakes were chosen from<br />
the Inyo National Forest, near Bishop, CA. These lakes<br />
included Lake Sabrina, Echo Lake, North Lake, TJ<br />
Lake, and Intake #1. The other five samples were<br />
taken from lakes in Orange County, Riverside County,<br />
and San Diego County. These included Laguna Niguel<br />
Lake, Irvine Lake, Lake Elsinore, Mission Viejo Lake,<br />
and Dixon Lake.<br />
Because the relative concentration <strong>of</strong><br />
phosphorous measured was so minute and the risk <strong>of</strong><br />
contamination was so high, care was taken to wash all<br />
glassware with phosphorous free soap. All chemicals<br />
and facilities were provided by the Chemistry<br />
Department at <strong>Saddleback</strong> <strong>College</strong>. The samples were<br />
decanted to remove any solid particles, and then<br />
standardized to 3.00 L. To make the testing and<br />
precipitating <strong>of</strong> phosphate more manageable, all <strong>of</strong> our<br />
samples were boiled down to approximately 40 mL, to<br />
be able to fit into a test tube. A 0.0010 M solution <strong>of</strong><br />
aluminum chloride was prepared and 5 mL were added<br />
to each <strong>of</strong> the samples to form a precipitate. To remove<br />
the possible interference <strong>of</strong> aluminum hydroxide, one<br />
drop <strong>of</strong> 0.100 M nitric acid solution was added to each<br />
<strong>of</strong> the samples. The samples were then centrifuged for<br />
15 minutes and the precipitates were decanted and<br />
washed. The washing and decanting process was<br />
repeated an additional three times to eliminate any<br />
extraneous dissolved ions. The resulting solutions were<br />
then placed into beakers <strong>of</strong> known weight and the<br />
excess water was boiled <strong>of</strong>f. The beakers were<br />
weighed again using an analytical balance, accurate to<br />
10 -4 grams, and the mass <strong>of</strong> the aluminum phosphate<br />
precipitate was determined. Accounting for the<br />
aluminum chloride added and considering that our<br />
original samples were 3.00 L, we converted the mass <strong>of</strong><br />
the precipitate into parts per billion <strong>of</strong> phosphorous.<br />
Results<br />
A one-tailed unpaired t-test was performed<br />
comparing the mean soluble phosphorous<br />
concentration for both urban and rural lake samples.<br />
The test yielded a p-value <strong>of</strong> 1.42x10 -4 , which shows<br />
that the urban lakes sampled had significantly higher<br />
levels <strong>of</strong> soluble phosphorous than the rural lakes<br />
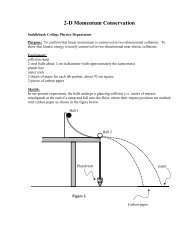
sampled (Figure 1).<br />
Soluble Phosphorous (ppb)<br />
10<br />
90<br />
8<br />
7<br />
6<br />
5<br />
4<br />
3<br />
2<br />
1<br />
0<br />
Urban Lake Location Rural<br />
Lake Location<br />
Figure 1. Mean soluble phosphorous in water samples<br />
from urban and rural lakes. The mean phosphorous<br />
concentration in urban lakes was 82.1589 ± 5.27461<br />
ppb (± SEM, n=5). The mean phosphorous<br />
concentration in rural lakes was 34.6547± 4.62362 ppb<br />
(± SEM, n=5). A one-tailed unpaired t-test revealed a<br />
significant difference between the phosphorous<br />
concentration in rural and urban lakes (P=1.42x10 -4 ).<br />
Figure 2. Carlson’s Trophic State Index which<br />
describes the degree <strong>of</strong> eutrophication in a lake based<br />
on the amount <strong>of</strong> total phosphorous in parts per billion.<br />
Discussion<br />
For further analysis <strong>of</strong> the eutrophication<br />
problem, the trophic state <strong>of</strong> the lakes was compared<br />
using The Carlson Trophic State Index (Figure 2). The<br />
mean phosphorous concentration <strong>of</strong> urban lakes, 82.16<br />
ppb, fell into the hypereutrophic zone. This rating<br />
describes a lake with excessive algal blooms, which<br />
has reduced oxygen content at lower depths and dead<br />
zones beneath the surface (Boesch et al., 2001). The<br />
rural lakes’ mean phosphorous concentration was 34.65<br />
ppb, falling into the mesotrophic to slightly eutrophic<br />
zone. Lakes in this classification are productive and<br />
support a regularly functioning ecosystem with a<br />
healthy balance between the primary producers and<br />
consumers.<br />
Our experiment also points to the possible<br />
efficacy <strong>of</strong> using aluminum to remove phosphate from<br />
a body <strong>of</strong> water. Our precipitate was obtained using<br />
very minute amounts <strong>of</strong> reagents and a slightly acidic<br />
solution, which mimics the natural condition <strong>of</strong> most<br />
lakes (Smith et al. 2001) Because aluminum phosphate<br />
127<br />
<strong>Saddleback</strong> <strong>Journal</strong> <strong>of</strong> <strong>Biology</strong><br />
Spring 2010