GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
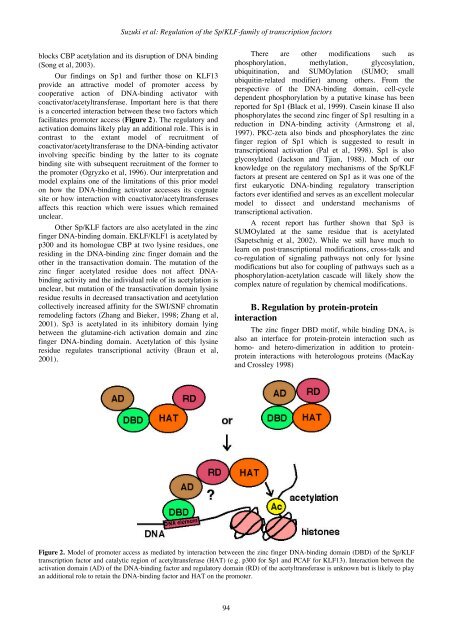
Suzuki et al: Regulation of the Sp/KLF-family of transcription factorsblocks CBP acetylation and its disruption of DNA binding(Song et al, 2003).Our findings on Sp1 and further those on KLF13provide an attractive model of promoter access bycooperative action of DNA-binding activator withcoactivator/acetyltransferase. Important here is that thereis a concerted interaction between these two factors whichfacilitates promoter access (Figure 2). The regulatory andactivation domains likely play an additional role. This is incontrast to the extant model of recruitment ofcoactivator/acetyltransferase to the DNA-binding activatorinvolving specific binding by the latter to its cognatebinding site with subsequent recruitment of the former tothe promoter (Ogryzko et al, 1996). Our interpretation andmodel explains one of the limitations of this prior modelon how the DNA-binding activator accesses its cognatesite or how interaction with coactivator/acetyltransferasesaffects this reaction which were issues which remainedunclear.Other Sp/KLF factors are also acetylated in the zincfinger DNA-binding domain. EKLF/KLF1 is acetylated byp300 and its homologue CBP at two lysine residues, oneresiding in the DNA-binding zinc finger domain and theother in the transactivation domain. The mutation of thezinc finger acetylated residue does not affect DNAbindingactivity and the individual role of its acetylation isunclear, but mutation of the transactivation domain lysineresidue results in decreased transactivation and acetylationcollectively increased affinity for the SWI/SNF chromatinremodeling factors (Zhang and Bieker, 1998; Zhang et al,2001). Sp3 is acetylated in its inhibitory domain lyingbetween the glutamine-rich activation domain and zincfinger DNA-binding domain. Acetylation of this lysineresidue regulates transcriptional activity (Braun et al,2001).There are other modifications such asphosphorylation, methylation, glycosylation,ubiquitination, and SUMOylation (SUMO; smallubiquitin-related modifier) among others. From theperspective of the DNA-binding domain, cell-cycledependent phosphorylation by a putative kinase has beenreported for Sp1 (Black et al, 1999). Casein kinase II alsophosphorylates the second zinc finger of Sp1 resulting in areduction in DNA-binding activity (Armstrong et al,1997). PKC-zeta also binds and phosphorylates the zincfinger region of Sp1 which is suggested to result intranscriptional activation (Pal et al, 1998). Sp1 is alsoglycosylated (Jackson and Tjian, 1988). Much of ourknowledge on the regulatory mechanisms of the Sp/KLFfactors at present are centered on Sp1 as it was one of thefirst eukaryotic DNA-binding regulatory transcriptionfactors ever identified and serves as an excellent molecularmodel to dissect and understand mechanisms oftranscriptional activation.A recent report has further shown that Sp3 isSUMOylated at the same residue that is acetylated(Sapetschnig et al, 2002). While we still have much tolearn on post-transcriptional modifications, cross-talk andco-regulation of signaling pathways not only for lysinemodifications but also for coupling of pathways such as aphosphorylation-acetylation cascade will likely show thecomplex nature of regulation by chemical modifications.B. Regulation by protein-proteininteractionThe zinc finger DBD motif, while binding DNA, isalso an interface for protein-protein interaction such ashomo- and hetero-dimerization in addition to proteinproteininteractions with heterologous proteins (MacKayand Crossley 1998)Figure 2. Model of promoter access as mediated by interaction betweeen the zinc finger DNA-binding domain (DBD) of the Sp/KLFtranscription factor and catalytic region of acetyltransferase (HAT) (e.g. p300 for Sp1 and PCAF for KLF13). Interaction between theactivation domain (AD) of the DNA-binding factor and regulatory domain (RD) of the acetyltransferase is unknown but is likely to playan additional role to retain the DNA-binding factor and HAT on the promoter.94