Jekunen et al: Strategy of sensitizing tumor cells with adenovirus-p53 transfectionthat CPT-11 and 5-fluorouracil may be useful asanticancer agents for use in a combination therapyregimen, using wild-type p53 gene transfer. These resultsindicate that CPT-11, as well as cisplatin, is a candidatefor the combination of chemotherapy and gene therapy forNSCLC. Adeno-p53 and DNA-damaging agents, cisplatin,etoposide and CPT-11 showed synergistic effects inNSCLC, but, in contrast had additive effects withantitubulin agents such as paclitaxel and docetaxel (Horio,Hasegawa et al, 2000). Perdomo et al, (Perdomo et al,1998) have demonstrated that human NSCLC cells havinga mutant form of p53 grow faster in vivo than wild-typep53 cell lines and the treatment with cisplatin or radiationdoes not reduce the size of mutant p53 tumors, althoughwild-type p53 tumors regress markedly. Apoptosisoccurred in mutant p53 cell types only at high cisplatindoses and not at the magnitude detected in wild-typetumors.III. In vivo evidence ofchemosensitization by adenovirus p53These observations have been extended to in vivomodels. Tumors have been treated in vivo withreplication-defective p53 adenovirus and chemotherapy.Nguyen et al, have reported convincing in vivo studies, inwhich p53null H1299 lung tumor xenografts were giveni.p. cisplatin before, concurrently with, or afterintratumoral adenovirus p53 (Nguyen et al, 1996). Themost effective dosing regimen was cisplatin given twodays before p53 therapy. Cisplatin and CPT-11 had asignificant antitumoral effect on lung cancer H157 cellxenografts of nude mice in vivo. Human head and neckcancer and colon cancer (Gjerset et al, 1997) and prostatecancer (Gjerset and Mercola 2000) in nude mice models invivo have been found to exhibit a similar sensitizationeffect with adenovirus plus cisplatin as in studies in vitro.Gjerset et al, demonstrated increased sensitivity tocisplatin cytotoxicity in p53mut T98G glioblastoma andp53 mut H23 small cell lung carcinoma cells transducedwith p53 expression vectors one or two days beforeexposure to cisplatin (Gjerset et al, 1995). These resultsare consistent with other in vivo studies in animal modelsshowing a combined benefit of p53 and chemotherapy(Badie et al, 1998), (Fujiwara et al, 1994), (Miyake et al,1998), (Nielsen et al, 1998), (Nguyen et al, 1996). Gjersetand Mercola are convinced that these results support theclinical application of adenovirus p53 combinationapproaches to tumors expressing mutant p53 (Gjerset andMercola 2000). Chemosensitization by p53 has also beenstudied using ex vivo modified cells in an orthotopicmodel of glioblastoma in Fisher rats (Dorigo et al, 1998).The combination of p53 with 5-fluorouracil andtopotecan has been studied in p53mut SW480 colorectaltumor cells transfected with an inducible p53 construct(Yang et al, 1996). Dose-dependent enhancement ofcytotoxicity was observed with these drugs by theconcurrent expression of wild-type p53. Increasedcytotoxicity has been reported in p53mut SkBr3 mammarytumor cells when transduction with p53 was followed 8 hrlater by doxorubicin or mitomycin-C, but not byvincristine (Blagosklonny and El-Deiry 1996).In the p53 null SK-OV-2 xenograft model of ovariancancer, a dosing schedule of the p53 therapy that, by itself,had a relatively minimal effect on the tumor burden (16%)caused a much greater decrease in tumor burden (55%)when combined with paclitaxel (Nielsen et al, 1998).Further, in nude mice implanted intraperitoneally with2774 human ovarian cancer cells (mutated p53), theresponse to adeno-p53 gene therapy showed significantsurvival duration, with a survival time greater than that ofuntreated animals. However, no statistically significantsurvival advantage was observed between adeno-p53- andadenovirus-βgal-treated mice (von Gruenigen et al, 1998).In another ovarian cancer study using nude mice, theadeno-p53 treatment effectively suppressed the growth ofperitoneal tumors and prolonged the survival of the treatedgroup, especially when the tumor burden was small (Kimet al, 1999). Greater combined efficacy was observed inthe p53null DU-145 prostate, p53Mut MDA-MB-468breast, and p53met MDA-MB-231 breast cancer xenograftmodels in vivo. The authors concluded that their data,taken together, offer the possibility of enhanced antitumoractivity with lower than normal doses of paclitaxel andadenovirus p53, when the two drugs are administered incombination (Nielsen et al, 1998). They noted that thiscould potentially decrease the chemotherapy-induced sideeffects, increasing the quality of life of the patients and,perhaps, reducing the overall expense of a complete courseof cancer treatment.IV. Clinical results of adenovirus p53transfection with chemotherapyThe first evidence of the efficacy of p53 gene therapyfor cancer was given by a pilot study in which retroviralp53 expression vectors were directly injected into smallendobronchial lesions of NSCLC patients (Roth et al,1996). Tumor regression was noted in three patients out ofnine, and tumor growth stabilized in three other patients.The safety and feasibility of the intratumoral injection ofadenoviral wild-type p53 expression vectors have beenestablished in NSCLC patients, with clear evidence fortransgenic expression, and possibly induction of apoptosis(Swisher et al, 1999; see Table 1). The antitumor activityin this trial was consistent with the activity of retroviralp53 injection in NSCLC patients. Twenty-four patientsreceived intratumor injections of adenovirus p53 and twopatients achieved a partial response, while 17 patientsachieved stable disease as the best clinical response.A nonrandomized, phase I, dose-escalating study byClayman et al expanded these findings into head and necksquamous cell carcinoma (Clayman et al, 1998). Patientswith incurable recurrent local or regionally metastaticHNSCC received multiple intratumoral injections ofadeno-p53, either with or without tumor resection. P53expression was detected in tumor biopsies despiteantibody responses after injections. prevent the appearanceof adeno-p53 in blood and urine. were seen in the study Asexpected, almost Neither dose-limiting effects nor serious30
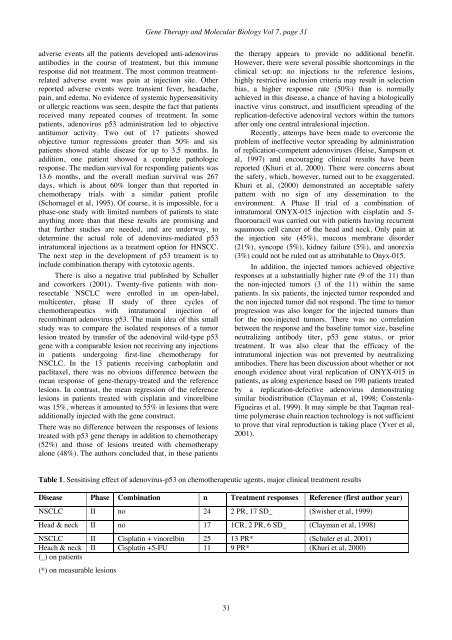
<strong>Gene</strong> <strong>Therapy</strong> and <strong>Molecular</strong> <strong>Biology</strong> Vol 7, page 31adverse events all the patients developed anti-adenovirusantibodies in the course of treatment, but this immuneresponse did not treatment. The most common treatmentrelatedadverse event was pain at injection site. Otherreported adverse events were transient fever, headache,pain, and edema. No evidence of systemic hypersensitivityor allergic reactions was seen, despite the fact that patientsreceived many repeated courses of treatment. In somepatients, adenovirus p53 administration led to objectiveantitumor activity. Two out of 17 patients showedobjective tumor regressions greater than 50% and sixpatients showed stable disease for up to 3.5 months. Inaddition, one patient showed a complete pathologicresponse. The median survival for responding patients was13.6 months, and the overall median survival was 267days, which is about 60% longer than that reported inchemotherapy trials with a similar patient profile(Schornagel et al, 1995). Of course, it is impossible, for aphase-one study with limited numbers of patients to stateanything more than that these results are promising andthat further studies are needed, and are underway, todetermine the actual role of adenovirus-mediated p53intratumoral injections as a treatment option for HNSCC.The next step in the development of p53 treament is toinclude combination therapy with cytotoxic agents.There is also a negative trial published by Schullerand coworkers (2001). Twenty-five patients with nonresectableNSCLC were enrolled in an open-label,multicenter, phase II study of three cycles ofchemotherapeutics with intratumoral injection ofrecombinant adenovirus p53. The main idea of this smallstudy was to compare the isolated responses of a tumorlesion treated by transfer of the adenoviral wild-type p53gene with a comparable lesion not receiving any injectionsin patients undergoing first-line chemotherapy forNSCLC. In the 13 patients receiving carboplatin andpaclitaxel, there was no obvious difference between themean response of gene-therapy-treated and the referencelesions. In contrast, the mean regression of the referencelesions in patients treated with cisplatin and vinorelbinewas 15%, whereas it amounted to 55% in lesions that wereadditionally injected with the gene construct.There was no difference between the responses of lesionstreated with p53 gene therapy in addition to chemotherapy(52%) and those of lesions treated with chemotherapyalone (48%). The authors concluded that, in these patientsthe therapy appears to provide no additional benefit.However, there were several possible shortcomings in theclinical set-up: no injections to the reference lesions,highly restrictive inclusion criteria may result in selectionbias, a higher response rate (50%) than is normallyachieved in this disease, a chance of having a biologicallyinactive virus construct, and insufficient spreading of thereplication-defective adenoviral vectors within the tumorsafter only one central intralesional injection.Recently, attemps have been made to overcome theproblem of ineffective vector spreading by administrationof replication-competent adenoviruses (Heise, Sampson etal, 1997) and encouraging clinical results have beenreported (Khuri et al, 2000). There were concerns aboutthe safety, which, however, turned out to be exaggerated.Khuri et al, (2000) demonstrated an acceptable safetypattern with no sign of any dissemination to theenvironment. A Phase II trial of a combination ofintratumoral ONYX-015 injection with cisplatin and 5-fluorouracil was carried out with patients having recurrentsquamous cell cancer of the head and neck. Only pain atthe injection site (45%), mucous membrane disorder(21%), syncope (5%), kidney failure (5%), and anorexia(3%) could not be ruled out as attributable to Onyx-015.In addition, the injected tumors achieved objectiveresponses at a substantially higher rate (9 of the 11) thanthe non-injected tumors (3 of the 11) within the samepatients. In six patients, the injected tumor responded andthe non injected tumor did not respond. The time to tumorprogression was also longer for the injected tumors thanfor the non-injected tumors. There was no correlationbetween the response and the baseline tumor size, baselineneutralizing antibody titer, p53 gene status, or priortreatment. It was also clear that the efficacy of theintratumoral injection was not prevented by neutralizingantibodies. There has been discussion about whether or notenough evidence about viral replication of ONYX-015 inpatients, as along experience based on 190 patients treatedby a replication-defective adenovirus demonstratingsimilar biodistribution (Clayman et al, 1998; Constenla-Figueiras et al, 1999). It may simple be that Taqman realtimepolymerase chain reaction technology is not sufficientto prove that viral reproduction is taking place (Yver et al,2001).Table 1. Sensitising effect of adenovirus-p53 on chemotherapeutic agents, major clinical treatment resultsDisease Phase Combination n Treatment responses Reference (first author year)NSCLC II no 24 2 PR, 17 SD_ (Swisher et al, 1999)Head & neck II no 17 1CR, 2 PR, 6 SD_ (Clayman et al, 1998)NSCLC II Cisplatin + vinorelbin 25 13 PR* (Schuler et al, 2001)Heach & neck II Cisplatin +5-FU 11 9 PR* (Khuri et al, 2000)(_) on patients(*) on measurable lesions31
- Page 7 and 8: Instructions to authors:Gene Therap
- Page 9: Please submit an electronic version
- Page 12: 103-111 ResearchArticle113-133 Revi
- Page 17 and 18: Gene Therapy and Molecular Biology
- Page 19 and 20: Gene Therapy and Molecular Biology
- Page 21 and 22: Gene Therapy and Molecular Biology
- Page 23 and 24: Gene Therapy and Molecular Biology
- Page 25 and 26: Gene Therapy and Molecular Biology
- Page 27 and 28: Gene Therapy and Molecular Biology
- Page 29 and 30: Gene Therapy and Molecular Biology
- Page 31 and 32: Gene Therapy and Molecular Biology
- Page 33 and 34: Gene Therapy and Molecular Biology
- Page 35 and 36: Gene Therapy and Molecular Biology
- Page 37 and 38: Gene Therapy and Molecular Biology
- Page 39 and 40: Gene Therapy and Molecular Biology
- Page 41 and 42: Gene Therapy and Molecular Biology
- Page 43: Gene Therapy and Molecular Biology
- Page 47 and 48: Gene Therapy and Molecular Biology
- Page 49 and 50: Gene Therapy and Molecular Biology
- Page 51 and 52: Gene Therapy and Molecular Biology
- Page 53 and 54: Gene Therapy and Molecular Biology
- Page 55 and 56: Gene Therapy and Molecular Biology
- Page 57 and 58: Gene Therapy and Molecular Biology
- Page 59 and 60: Gene Therapy and Molecular Biology
- Page 61 and 62: Gene Therapy and Molecular Biology
- Page 63 and 64: Gene Therapy and Molecular Biology
- Page 65 and 66: Gene Therapy and Molecular Biology
- Page 67 and 68: Gene Therapy and Molecular Biology
- Page 69 and 70: Gene Therapy and Molecular Biology
- Page 71 and 72: Gene Therapy and Molecular Biology
- Page 73 and 74: Gene Therapy and Molecular Biology
- Page 75 and 76: Gene Therapy and Molecular Biology
- Page 77: Gene Therapy and Molecular Biology
- Page 80 and 81: Epperly et al: Late injection of Mn
- Page 82 and 83: Epperly et al: Late injection of Mn
- Page 84 and 85: Goldberg-Cohen et al: Regulation of
- Page 86 and 87: Goldberg-Cohen et al: Regulation of
- Page 88 and 89: Goldberg-Cohen et al: Regulation of
- Page 90 and 91: Gascón-Irún et al: Gene therapy a
- Page 92 and 93: Gascón-Irún et al: Gene therapy a
- Page 94 and 95:
Gascón-Irún et al: Gene therapy a
- Page 96 and 97:
Gascón-Irún et al: Gene therapy a
- Page 98 and 99:
Gascón-Irún et al: Gene therapy a
- Page 100 and 101:
Gascón-Irún et al: Gene therapy a
- Page 102 and 103:
Gascón-Irún et al: Gene therapy a
- Page 104 and 105:
Gascón-Irún et al: Gene therapy a
- Page 106 and 107:
Suzuki et al: Regulation of the Sp/
- Page 108 and 109:
Suzuki et al: Regulation of the Sp/
- Page 110 and 111:
Suzuki et al: Regulation of the Sp/
- Page 112 and 113:
Suzuki et al: Regulation of the Sp/
- Page 114 and 115:
Li et al: MET amplification in live
- Page 116 and 117:
Li et al: MET amplification in live
- Page 118 and 119:
Chavakis et al: Leukocyte adhesion
- Page 120 and 121:
Chavakis et al: Leukocyte adhesion
- Page 122 and 123:
Chavakis et al: Leukocyte adhesion
- Page 124 and 125:
Chavakis et al: Leukocyte adhesion
- Page 126 and 127:
Chavakis et al: Leukocyte adhesion
- Page 128 and 129:
Sanlioglu et al: Adenovirus mediate
- Page 130 and 131:
Sanlioglu et al: Adenovirus mediate
- Page 132 and 133:
Sanlioglu et al: Adenovirus mediate
- Page 134 and 135:
Sanlioglu et al: Adenovirus mediate
- Page 136 and 137:
Sanlioglu et al: Adenovirus mediate
- Page 138 and 139:
Sanlioglu et al: Adenovirus mediate
- Page 140 and 141:
Sanlioglu et al: Adenovirus mediate
- Page 142 and 143:
Sanlioglu et al: Adenovirus mediate
- Page 144 and 145:
Sanlioglu et al: Adenovirus mediate
- Page 146 and 147:
Sanlioglu et al: Adenovirus mediate
- Page 148 and 149:
Sanlioglu et al: Adenovirus mediate
- Page 150 and 151:
George et al: Gene therapy for vasc
- Page 152 and 153:
George et al: Gene therapy for vasc
- Page 154 and 155:
George et al: Gene therapy for vasc
- Page 156 and 157:
George et al: Gene therapy for vasc
- Page 158 and 159:
George et al: Gene therapy for vasc
- Page 160 and 161:
George et al: Gene therapy for vasc
- Page 162 and 163:
George et al: Gene therapy for vasc
- Page 164 and 165:
George et al: Gene therapy for vasc
- Page 166 and 167:
George et al: Gene therapy for vasc
- Page 168 and 169:
Zhang et al: Angiogenic Gene Therap
- Page 170 and 171:
Zhang et al: Angiogenic Gene Therap
- Page 172 and 173:
Zhang et al: Angiogenic Gene Therap
- Page 174 and 175:
Zhang et al: Angiogenic Gene Therap
- Page 176 and 177:
Zhang et al: Angiogenic Gene Therap
- Page 178 and 179:
Zhang et al: Angiogenic Gene Therap
- Page 180 and 181:
Zhang et al: Angiogenic Gene Therap
- Page 182 and 183:
Xu et al: G-CSF receptor-mediated S
- Page 184 and 185:
Xu et al: G-CSF receptor-mediated S
- Page 186 and 187:
Xu et al: G-CSF receptor-mediated S
- Page 188 and 189:
Burek et al: Calcium induced cell d
- Page 190 and 191:
Burek et al: Calcium induced cell d
- Page 192 and 193:
Burek et al: Calcium induced cell d
- Page 194 and 195:
Burek et al: Calcium induced cell d
- Page 196 and 197:
David et al: Current status and fut
- Page 198 and 199:
David et al: Current status and fut
- Page 200 and 201:
David et al: Current status and fut
- Page 202 and 203:
David et al: Current status and fut
- Page 204 and 205:
David et al: Current status and fut
- Page 206 and 207:
David et al: Current status and fut
- Page 208 and 209:
David et al: Current status and fut
- Page 210 and 211:
David et al: Current status and fut
- Page 212 and 213:
David et al: Current status and fut
- Page 214 and 215:
David et al: Current status and fut
- Page 216 and 217:
David et al: Current status and fut
- Page 218 and 219:
David et al: Current status and fut
- Page 220 and 221:
David et al: Current status and fut
- Page 222 and 223:
David et al: Current status and fut
- Page 224 and 225:
David et al: Current status and fut
- Page 226 and 227:
Stoll et al: The role of EBV and ge
- Page 228 and 229:
Stoll et al: The role of EBV and ge
- Page 230 and 231:
Stoll et al: The role of EBV and ge
- Page 232 and 233:
Stoll et al: The role of EBV and ge
- Page 234 and 235:
Stoll et al: The role of EBV and ge
- Page 236 and 237:
Maruyama et al: Kidney-targeted pla
- Page 238 and 239:
Maruyama et al: Kidney-targeted pla
- Page 240 and 241:
Maruyama et al: Kidney-targeted pla
- Page 242 and 243:
Maruyama et al: Kidney-targeted pla
- Page 244 and 245:
Kren et al: Hepatocyte-targeted del
- Page 246 and 247:
Kren et al: Hepatocyte-targeted del
- Page 248 and 249:
Kren et al: Hepatocyte-targeted del
- Page 250 and 251:
Kren et al: Hepatocyte-targeted del
- Page 252 and 253:
Kren et al: Hepatocyte-targeted del
- Page 254 and 255:
Zeng: PRL-3 as a target for cancer
- Page 256 and 257:
Zeng: PRL-3 as a target for cancer
- Page 258 and 259:
Zeng: PRL-3 as a target for cancer
- Page 260 and 261:
Latchman: Protective effect of heat
- Page 262 and 263:
Latchman: Protective effect of heat
- Page 264 and 265:
Latchman: Protective effect of heat
- Page 266 and 267:
Latchman: Protective effect of heat
- Page 268 and 269:
Latchman: Protective effect of heat
- Page 270 and 271:
Cai et al: Lung cancer gene therapy
- Page 272 and 273:
Cai et al: Lung cancer gene therapy
- Page 274 and 275:
Cai et al: Lung cancer gene therapy
- Page 276 and 277:
Cai et al: Lung cancer gene therapy
- Page 278 and 279:
Cai et al: Lung cancer gene therapy
- Page 280:
Cai et al: Lung cancer gene therapy
- Page 283 and 284:
Gene Therapy and Molecular Biology
- Page 285 and 286:
Gene Therapy and Molecular Biology
- Page 287 and 288:
Gene Therapy and Molecular Biology
- Page 289 and 290:
Gene Therapy and Molecular Biology
- Page 291 and 292:
Gene Therapy and Molecular Biology
- Page 293 and 294:
Gene Therapy and Molecular Biology
- Page 295 and 296:
Gene Therapy and Molecular Biology
- Page 297 and 298:
Gene Therapy and Molecular Biology
- Page 299 and 300:
Gene Therapy and Molecular Biology
- Page 301 and 302:
Gene Therapy and Molecular Biology
- Page 303 and 304:
Gene Therapy and Molecular Biology
- Page 305 and 306:
Gene Therapy and Molecular Biology
- Page 307 and 308:
Gene Therapy and Molecular Biology
- Page 309:
Gene Therapy and Molecular Biology