GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
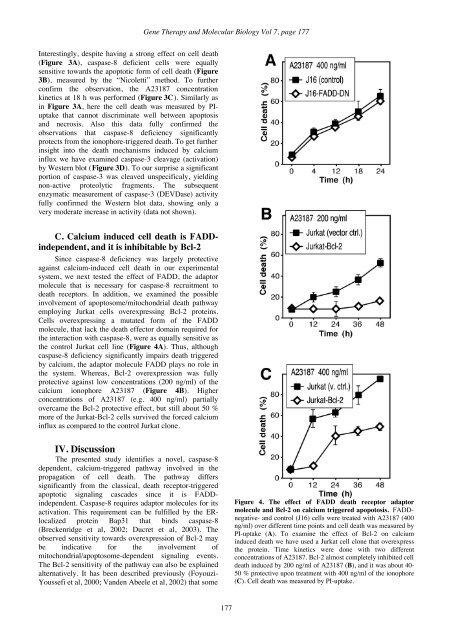
<strong>Gene</strong> <strong>Therapy</strong> and <strong>Molecular</strong> <strong>Biology</strong> Vol 7, page 177Interestingly, despite having a strong effect on cell death(Figure 3A), caspase-8 deficient cells were equallysensitive towards the apoptotic form of cell death (Figure3B), measured by the “Nicoletti” method. To furtherconfirm the observation, the A23187 concentrationkinetics at 18 h was performed (Figure 3C). Similarly asin Figure 3A, here the cell death was measured by PIuptakethat cannot discriminate well between apoptosisand necrosis. Also this data fully confirmed theobservations that caspase-8 deficiency significantlyprotects from the ionophore-triggered death. To get furtherinsight into the death mechanisms induced by calciuminflux we have examined caspase-3 cleavage (activation)by Western blot (Figure 3D). To our surprise a significantportion of caspase-3 was cleaved unspecificaly, yieldingnon-active proteolytic fragments. The subsequentenzymatic measurement of caspase-3 (DEVDase) activityfully confirmed the Western blot data, showing only avery moderate increase in activity (data not shown).C. Calcium induced cell death is FADDindependent,and it is inhibitable by Bcl-2Since caspase-8 deficiency was largely protectiveagainst calcium-induced cell death in our experimentalsystem, we next tested the effect of FADD, the adaptormolecule that is necessary for caspase-8 recruitment todeath receptors. In addition, we examined the possibleinvolvement of apoptosome/mitochondrial death pathwayemploying Jurkat cells overexpressing Bcl-2 proteins.Cells overexpressing a mutated form of the FADDmolecule, that lack the death effector domain required forthe interaction with caspase-8, were as equally sensitive asthe control Jurkat cell line (Figure 4A). Thus, althoughcaspase-8 deficiency significantly impairs death triggeredby calcium, the adaptor molecule FADD plays no role inthe system. Whereas, Bcl-2 overexpression was fullyprotective against low concentrations (200 ng/ml) of thecalcium ionophore A23187 (Figure 4B). Higherconcentrations of A23187 (e.g. 400 ng/ml) partiallyovercame the Bcl-2 protective effect, but still about 50 %more of the Jurkat-Bcl-2 cells survived the forced calciuminflux as compared to the control Jurkat clone.IV. DiscussionThe presented study identifies a novel, caspase-8dependent, calcium-triggered pathway involved in thepropagation of cell death. The pathway differssignificantly from the classical, death receptor-triggeredapoptotic signaling cascades since it is FADDindependent.Caspase-8 requires adaptor molecules for itsactivation. This requirement can be fulfilled by the ERlocalizedprotein Bap31 that binds caspase-8(Breckenridge et al, 2002; Ducret et al, 2003). Theobserved sensitivity towards overexpression of Bcl-2 maybe indicative for the involvement ofmitochondrial/apoptosome-dependent signaling events.The Bcl-2 sensitivity of the pathway can also be explainedalternatively. It has been described previously (Foyouzi-Youssefi et al, 2000; Vanden Abeele et al, 2002) that someFigure 4. The effect of FADD death receptor adaptormolecule and Bcl-2 on calcium triggered apopotosis. FADDnegative-and control (J16) cells were treated with A23187 (400ng/ml) over different time points and cell death was measured byPI-uptake (A). To examine the effect of Bcl-2 on calciuminduced death we have used a Jurkat cell clone that overexpressthe protein. Time kinetics were done with two differentconcentrations of A23187. Bcl-2 almost completely inhibited celldeath induced by 200 ng/ml of A23187 (B), and it was about 40-50 % protective upon treatment with 400 ng/ml of the ionophore(C). Cell death was measured by PI-uptake.177