Zhang et al: Angiogenic <strong>Gene</strong> <strong>Therapy</strong> for Improving Islet Graft Vascularizationkidneys by diabetic nephropathy (Chaturvedi et al, 2000)and on nerves by diabetic neuropathy, together with about4- and 10-fold lifetime increase in rates of cardiovascularmortality among men and women, respectively (Laing etal, 2003). To improve glycemic control, a number ofinsulin analogs, such as short-acting insulin lispro andaspart (Plank et al, 2002), as well as delayed-acting insulinglargine (Murphy et al, 2003) and detimir (Vague et al,2003) have been developed. Nevertheless, implementationof treatment regimens with insulin analogs in differentformulations to strive for normoglycemic control withoutrisk of hypoglycemia can be very challenging and requiresextraordinary efforts from both health care providers anddiabetic patients (Bloomgarden et al, 2002).sources by generating insulin-producing cells throughgenetic engineering of embryonic stem cells (Lumelsky etal, 2001; Soria et al, 2001). In addition, limited progresshas been made to induce graft tolerance using immunemodulation or allorecognition (Cote et al, 2001). An indepthdiscussion of these two outstanding issues inrelation to the optimal clinical outcome of islettransplantation, which is beyond the scope of this article,has been reviewed elsewhere (Waldmann, 2002; Lechleret al, 2003; Lechner and Habener, 2003). Here we wouldlike to highlight a third limiting factor, namely isletrevascularization, which appears to play an important rolein determining the long-term survival and optimalperformance of functional islet mass post transplantation.C. Islet transplantationOf alternative insulin replacement therapiesdeveloped, islet transplantation offers the prospect ofproviding a curative treatment for type 1 diabetes withoutthe need for exogenous insulin. The protocol of islettransplantation developed by Shapiro and colleagues at theUniversity of Alberta at Edmonton, Canada, known as theEdmonton protocol, is relatively simple and minimallyinvasive, which is carried out under local anestheticswithout surgery. Using fluoroscopic guidance, isolatedhuman islets are implanted intraportally to a diabeticrecipient, such that islets are engrafted in the liver andfunction to provide near physiological insulin release froman ectopic site. The success of this protocol has largelybeen attributed to technical advances in isolating highqualityhuman islets in relatively large quantities and theapplication of more potent and less toxic non-steroidalimmunosuppressants (Shapiro et al, 2000). Using theEdmonton protocol, long-term excellent glycemic controlhas been achieved with sustained freedom from insulininjection in type 1 diabetic patients (Shapiro et al, 2000).Currently, this protocol is being rigorously tested inclinical trials at multiple clinical centers to evaluate thesafety and efficacy of islet transplantation and assess thebenefit and risk ratio associated with long-term use ofimmunosuppressive drugs (Boker et al, 2001).Although promising for providing a curative optionfor type 1 diabetes, the Edmonton protocol is limited bytwo major factors: the lack of a sufficiently large source ofislets due to the scarcity of cadaveric pancreas donors, andthe presence of persistent immune rejection as well as thepotential for recurrence of autoimmunity. Recent followupstudies indicate that even with the rigorous applicationof steroid-free immunosuppressive regimens, there is stilla slow and progressive loss of insulin production fromtransplanted islets in diabetic recipients over time, asevidenced by reports that 30-40% of islet recipients mayexperience recurrence of autoimmune diabetes with reacquisitionof insulin dependence one to two years posttransplantation (Shapiro et al, 2000; Boker et al, 2001;Ryan et al, 2001, 2002). To overcome these limitations,attempts have been made to develop alternative islet1. Islet revascularization posttransplantationa. Re-establishment of islet microvasculature.Native islets in the pancreas have a rich glomerularlikevascular system that consists of fine capillariessupplied by one to five feeding arterioles and drained bycoalescing into an efferent plexus exiting the islet via oneto five venules (Menger et al, 2001; Mattson et al, 2002).Such a rich microvasculature in pancreatic islets serves toprovide efficient delivery of oxygen and nutrients to isletcells, and at the same time ensure rapid dispersal ofpancreatic hormones to the circulation. However, isolatedislets are avascular in both structural and functionalentities, such that after transplantation, the survival andfunction of islets must rely on the re-establishment of newvessels in the grafts to derive blood flow from the hostvessel system (Boker et al, 2001; Vasir et al, 2001). Thereis evidence that freely transplanted islets are associatedwith significantly reduced islet revascularization incomparison to native islets in the pancreas and thisproblem occurs irrespective of whether islets aretransplanted intraportally in the liver, retrogradely into thespleen, or under the kidney capsule (Figure 1) (Mattson etal, 2002).What are the likely consequences of delayed orinsufficient islet revascularization post islettransplantation? To answer this question, let us take aquantitative view of the relative partitioning of blood flowto islets vs. exocrine tissue in the pancreas. Using amodified microsphere technique, it has been shown thatislets take up more than 10% of the total pancreatic bloodflow despite their collectively comprising only about 1%of the tissue mass of the pancreas (Jansson and Carlsson,2002). Thus, it is critically important to maintain adequatemicrovascular perfusion to islet cells for oxygen andnutrient supplies. While islets are transplanted either assingle entities or as aggregated islet clusters under thekidney capsule or intraportally in the liver, adequatemicrovascular perfusion to islet cells does not resumeimmediately after islet transplantation.154
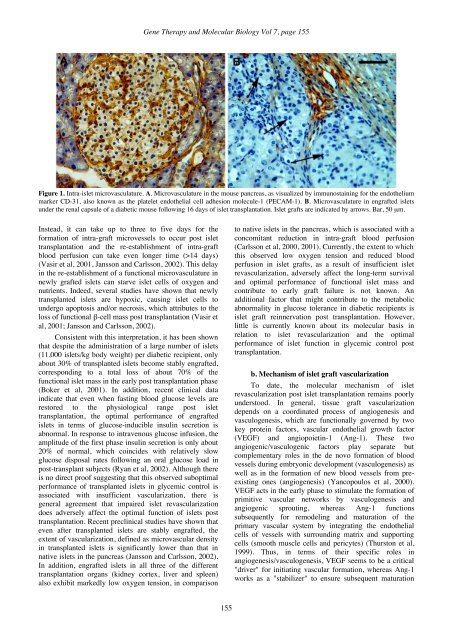
<strong>Gene</strong> <strong>Therapy</strong> and <strong>Molecular</strong> <strong>Biology</strong> Vol 7, page 155Figure 1. Intra-islet microvasculature. A. Microvasculature in the mouse pancreas, as visualized by immunostaining for the endotheliummarker CD-31, also known as the platelet endothelial cell adhesion molecule-1 (PECAM-1). B. Microvasculature in engrafted isletsunder the renal capsule of a diabetic mouse following 16 days of islet transplantation. Islet grafts are indicated by arrows. Bar, 50 µm.Instead, it can take up to three to five days for theformation of intra-graft microvessels to occur post islettransplantation and the re-establishment of intra-graftblood perfusion can take even longer time (>14 days)(Vasir et al, 2001, Jansson and Carlsson, 2002). This delayin the re-establishment of a functional microvasculature innewly grafted islets can starve islet cells of oxygen andnutrients. Indeed, several studies have shown that newlytransplanted islets are hypoxic, causing islet cells toundergo apoptosis and/or necrosis, which attributes to theloss of functional β-cell mass post transplantation (Vasir etal, 2001; Jansson and Carlsson, 2002).Consistent with this interpretation, it has been shownthat despite the administration of a large number of islets(11,000 islets/kg body weight) per diabetic recipient, onlyabout 30% of transplanted islets become stably engrafted,corresponding to a total loss of about 70% of thefunctional islet mass in the early post transplantation phase(Boker et al, 2001). In addition, recent clinical dataindicate that even when fasting blood glucose levels arerestored to the physiological range post islettransplantation, the optimal performance of engraftedislets in terms of glucose-inducible insulin secretion isabnormal. In response to intravenous glucose infusion, theamplitude of the first phase insulin secretion is only about20% of normal, which coincides with relatively slowglucose disposal rates following an oral glucose load inpost-transplant subjects (Ryan et al, 2002). Although thereis no direct proof suggesting that this observed suboptimalperformance of transplanted islets in glycemic control isassociated with insufficient vascularization, there isgeneral agreement that impaired islet revascularizationdoes adversely affect the optimal function of islets posttransplantation. Recent preclinical studies have shown thateven after transplanted islets are stably engrafted, theextent of vascularization, defined as microvascular densityin transplanted islets is significantly lower than that innative islets in the pancreas (Jansson and Carlsson, 2002).In addition, engrafted islets in all three of the differenttransplantation organs (kidney cortex, liver and spleen)also exhibit markedly low oxygen tension, in comparisonto native islets in the pancreas, which is associated with aconcomitant reduction in intra-graft blood perfusion(Carlsson et al, 2000, 2001). Currently, the extent to whichthis observed low oxygen tension and reduced bloodperfusion in islet grafts, as a result of insufficient isletrevascularization, adversely affect the long-term survivaland optimal performance of functional islet mass andcontribute to early graft failure is not known. Anadditional factor that might contribute to the metabolicabnormality in glucose tolerance in diabetic recipients isislet graft reinnervation post transplantation. However,little is currently known about its molecular basis inrelation to islet revascularization and the optimalperformance of islet function in glycemic control posttransplantation.b. Mechanism of islet graft vascularizationTo date, the molecular mechanism of isletrevascularization post islet transplantation remains poorlyunderstood. In general, tissue graft vascularizationdepends on a coordinated process of angiogenesis andvasculogenesis, which are functionally governed by twokey protein factors, vascular endothelial growth factor(VEGF) and angiopoietin-1 (Ang-1). These twoangiogenic/vasculogenic factors play separate butcomplementary roles in the de novo formation of bloodvessels during embryonic development (vasculogenesis) aswell as in the formation of new blood vessels from preexistingones (angiogenesis) (Yancopoulos et al, 2000).VEGF acts in the early phase to stimulate the formation ofprimitive vascular networks by vasculogenesis andangiogenic sprouting, whereas Ang-1 functionssubsequently for remodeling and maturation of theprimary vascular system by integrating the endothelialcells of vessels with surrounding matrix and supportingcells (smooth muscle cells and pericytes) (Thurston et al,1999). Thus, in terms of their specific roles inangiogenesis/vasculogenesis, VEGF seems to be a critical"driver" for initiating vascular formation, whereas Ang-1works as a "stabilizer" to ensure subsequent maturation155