GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
GTMB 7 - Gene Therapy & Molecular Biology
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
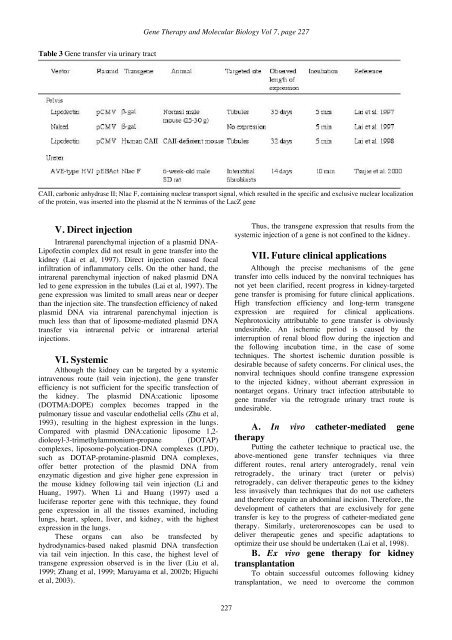
<strong>Gene</strong> <strong>Therapy</strong> and <strong>Molecular</strong> <strong>Biology</strong> Vol 7, page 227Table 3 <strong>Gene</strong> transfer via urinary tractCAII, carbonic anhydrase II; Nlac F, containing nuclear transport signal, which resulted in the specific and exclusive nuclear localizationof the protein, was inserted into the plasmid at the N terminus of the LacZ geneV. Direct injectionIntrarenal parenchymal injection of a plasmid DNA-Lipofectin complex did not result in gene transfer into thekidney (Lai et al, 1997). Direct injection caused focalinfiltration of inflammatory cells. On the other hand, theintrarenal parenchymal injection of naked plasmid DNAled to gene expression in the tubules (Lai et al, 1997). Thegene expression was limited to small areas near or deeperthan the injection site. The transfection efficiency of nakedplasmid DNA via intrarenal parenchymal injection ismuch less than that of liposome-mediated plasmid DNAtransfer via intrarenal pelvic or intrarenal arterialinjections.VI. SystemicAlthough the kidney can be targeted by a systemicintravenous route (tail vein injection), the gene transferefficiency is not sufficient for the specific transfection ofthe kidney. The plasmid DNA:cationic liposome(DOTMA:DOPE) complex becomes trapped in thepulmonary tissue and vascular endothelial cells (Zhu et al,1993), resulting in the highest expression in the lungs.Compared with plasmid DNA:cationic liposome 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP)complexes, liposome-polycation-DNA complexes (LPD),such as DOTAP-protamine-plasmid DNA complexes,offer better protection of the plasmid DNA fromenzymatic digestion and give higher gene expression inthe mouse kidney following tail vein injection (Li andHuang, 1997). When Li and Huang (1997) used aluciferase reporter gene with this technique, they foundgene expression in all the tissues examined, includinglungs, heart, spleen, liver, and kidney, with the highestexpression in the lungs.These organs can also be transfected byhydrodynamics-based naked plasmid DNA transfectionvia tail vein injection. In this case, the highest level oftransgene expression observed is in the liver (Liu et al,1999; Zhang et al, 1999; Maruyama et al, 2002b; Higuchiet al, 2003).Thus, the transgene expression that results from thesystemic injection of a gene is not confined to the kidney.VII. Future clinical applicationsAlthough the precise mechanisms of the genetransfer into cells induced by the nonviral techniques hasnot yet been clarified, recent progress in kidney-targetedgene transfer is promising for future clinical applications.High transfection efficiency and long-term transgeneexpression are required for clinical applications.Nephrotoxicity attributable to gene transfer is obviouslyundesirable. An ischemic period is caused by theinterruption of renal blood flow during the injection andthe following incubation time, in the case of sometechniques. The shortest ischemic duration possible isdesirable because of safety concerns. For clinical uses, thenonviral techniques should confine transgene expressionto the injected kidney, without aberrant expression innontarget organs. Urinary tract infection attributable togene transfer via the retrograde urinary tract route isundesirable.A. In vivo catheter-mediated genetherapyPutting the catheter technique to practical use, theabove-mentioned gene transfer techniques via threedifferent routes, renal artery anterogradely, renal veinretrogradely, the urinary tract (ureter or pelvis)retrogradely, can deliver therapeutic genes to the kidneyless invasively than techniques that do not use cathetersand therefore require an abdominal incision. Therefore, thedevelopment of catheters that are exclusively for genetransfer is key to the progress of catheter-mediated genetherapy. Similarly, ureterorenoscopes can be used todeliver therapeutic genes and specific adaptations tooptimize their use should be undertaken (Lai et al, 1998).B. Ex vivo gene therapy for kidneytransplantationTo obtain successful outcomes following kidneytransplantation, we need to overcome the common227