CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Gold Nanorings from Block Copolymer Self-assembly<br />
L. Wang, P. Hoffmann • , R. Pugin, F. Montagne<br />
<strong>CSEM</strong> presents a simple approach for the synthesis of long range ordered arrays of gold nanorings by exploiting the stimuli-responsive properties<br />
of amphiphilic block copolymer micelles. The size <strong>and</strong> periodicity of the nanoring array can be tuned by adjusting the dimensions of the parent<br />
micelles. A mechanism for the nanoring formation based on a pH-dependent kinetic competition between micelle phase inversion <strong>and</strong> metal loading<br />
has been proposed. Preliminary surface plasmon resonance (SPR) indicated significant change in the optical properties compared to solid gold<br />
nanoparticles, opening the door to interesting applications in sensing. This work demonstrates the ability to achieve precise positioning of metallic<br />
nanostructures on semiconductor surfaces using self-assembly processes.<br />
Surface patterning at the nanometer scale is attracting<br />
considerable attention due to its enormous potential in the<br />
design of surfaces exhibiting radically new functionalities. For<br />
instance, the possibility to form regular arrays of metallic<br />
nanostructures on semiconductor surfaces opens a wide<br />
spectrum of applications in electronics, photonics <strong>and</strong><br />
biosensing. Advanced top-down nanolithography techniques<br />
(e.g. deep UV, e-beam, focused ion beam) allow to produce<br />
feature sizes of less than 100 nm, but become increasingly<br />
costly as the dimensions move further down towards the<br />
10 nm mark.<br />
In this context, the synthesis of metallic nanostructures <strong>and</strong><br />
the control of their spatial arrangement at the nanoscale by<br />
means of block copolymer (BCP) self-assembly are a very<br />
attractive <strong>and</strong> powerful alternative. Here, for the first time,<br />
<strong>CSEM</strong> exploits the responsive properties of BCP micelles to<br />
form long range <strong>and</strong> tunable arrays of ring-shaped gold<br />
nanostructures having feature sizes below 100 nm.<br />
To produce the gold nanorings, a silicon substrate coated with<br />
reverse polystyrene-block-poly (2-vinylpyridine) (PS-b-P2VP)<br />
micelles is immersed in an aqueous tetrachloroauric solution<br />
(1 mM HAuCl4/0.9 wt% HCl (aq.)) for several minutes. It is<br />
then rinsed with deionised water, dried under a nitrogen<br />
stream <strong>and</strong> exposed to oxygen plasma in order to<br />
simultaneously remove the surrounding polymer <strong>and</strong> reduce<br />
the metal salt to Au(0). Figure 1 presents two SEM images (at<br />
different magnifications) of gold nanorings having sizes (outer<br />
diameter) around 50 nm.<br />
Figure 1: SEM images of continuous gold nanorings obtained by<br />
immersion of the reverse micelles in 1 mM HAuCl4/0.9 wt% HCl (aq.)<br />
solution for 10 min, followed by oxygen plasma exposure.<br />
Interestingly, only observed the formation of the gold<br />
nanorings at low pH was observed. Indeed, in aqueous acidic<br />
conditions, <strong>and</strong> ideally at a pH value ≤ pKa - 2 (pKa of<br />
pyridine is ∼5.2), the P2VP blocks are rapidly swollen <strong>and</strong><br />
protonated <strong>and</strong> thus carry a net positive charge which allow<br />
them to strongly bind anionic metal species through the<br />
establishment of electrostatic interactions. Since at low pH<br />
values, the phase inversion is faster than metal coordination,<br />
[AuCl4] - will interact with the cationic polymeric micelles.<br />
Subsequent treatment under oxygen plasma will then reduce<br />
the metal salt <strong>and</strong> remove the block copolymer leading to the<br />
formation of the gold nanoring array. Obviously, this<br />
mechanism excludes the synthesis of nanorings from cationic<br />
metallic species (eg. Ag + ) since repulsive interaction with the<br />
protonated pyridine group would prevent metal binding.<br />
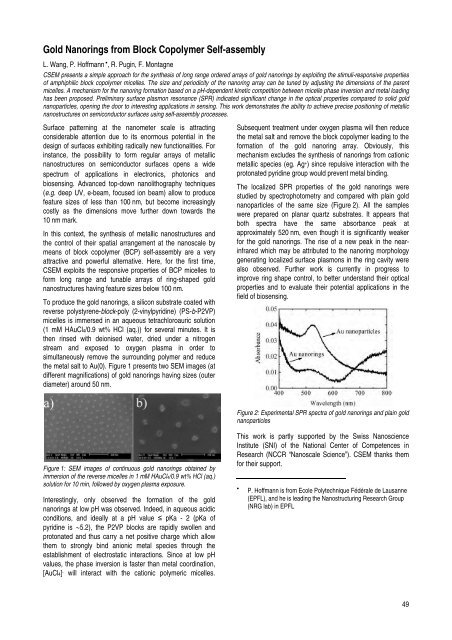
The localized SPR properties of the gold nanorings were<br />
studied by spectrophotometry <strong>and</strong> compared with plain gold<br />
nanoparticles of the same size (Figure 2). All the samples<br />
were prepared on planar quartz substrates. It appears that<br />
both spectra have the same absorbance peak at<br />
approximately 520 nm, even though it is significantly weaker<br />
for the gold nanorings. The rise of a new peak in the nearinfrared<br />
which may be attributed to the nanoring morphology<br />
generating localized surface plasmons in the ring cavity were<br />
also observed. Further work is currently in progress to<br />
improve ring shape control, to better underst<strong>and</strong> their optical<br />
properties <strong>and</strong> to evaluate their potential applications in the<br />
field of biosensing.<br />
Figure 2: Experimental SPR spectra of gold nanorings <strong>and</strong> plain gold<br />
nanoparticles<br />
This work is partly supported by the Swiss Nanoscience<br />
Institute (SNI) of the National Center of Competences in<br />
Research (NCCR “Nanoscale Science”). <strong>CSEM</strong> thanks them<br />
for their support.<br />
• P. Hoffmann is from Ecole Polytechnique Fédérale de Lausanne<br />
(EPFL), <strong>and</strong> he is leading the Nanostructuring Research Group<br />
(NRG lab) in EPFL<br />
49