CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
Surfaces that Regulate Cell Growth <strong>and</strong> Death<br />
M. Giazzon, N. Blondiaux, N. Matthey, G. Weder, M. Liley<br />
Microstructured silicon surfaces have been used as substrates for bone cell growth. Cell proliferation <strong>and</strong> death can be regulated by varying the<br />
height of the microstructures while leaving the other dimensions unchanged. These changes in the surface structure are accompanied by a change<br />
in cell morphology from a 2D shape to a 3D one. This involves modification of the cytoskeleton <strong>and</strong> a progressive loss of cell-surface adhesion.<br />
Osteoblasts are cells specialized in the production of bone<br />
tissue. They synthesize the two components of bone matrix: a<br />
fibrous matrix that bestows elasticity on the bone <strong>and</strong> an<br />
amorphous one that provides hardness <strong>and</strong> resistance to<br />
compression. The correct behaviour of osteoblasts is crucial<br />
to the successful integration of bone <strong>and</strong> tooth implants. For<br />
this reason there is an increasing effort focused on the search<br />
for new substrates which can mimic the environment that<br />
osteoblasts create around themselves <strong>and</strong> induce implant<br />
integration.<br />
At <strong>CSEM</strong>, studies of silicon surfaces with pillar structures,<br />
together with their capacity to influence osteoblast behaviour,<br />
including cell growth, death <strong>and</strong> morphology are underway [1] .<br />
The surfaces were fabricated by dry etching, using a<br />
structured polymer thin film as mask. By varying the etch time,<br />
different pillar heights could be created while the diameter<br />
(~ 400 nm) <strong>and</strong> the spacing of the pillars (~ 700 nm) remained<br />
unchanged (Figure 1). This fabrication method has a<br />
significant advantage in that it allows the controlled variation<br />
of the topography while leaving the surface chemistry<br />
unchanged.<br />
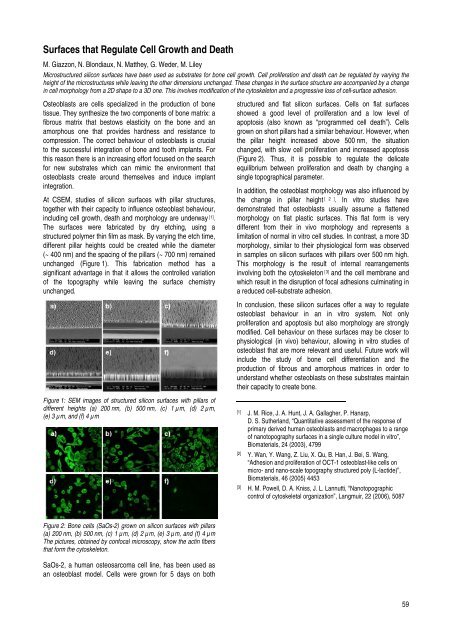
Figure 1: SEM images of structured silicon surfaces with pillars of<br />
different heights (a) 200 nm, (b) 500 nm, (c) 1 µm, (d) 2 µm,<br />
(e) 3 µm, <strong>and</strong> (f) 4 µm<br />
Figure 2: Bone cells (SaOs-2) grown on silicon surfaces with pillars<br />
(a) 200 nm, (b) 500 nm, (c) 1 µm, (d) 2 µm, (e) 3 µm, <strong>and</strong> (f) 4 µm<br />
The pictures, obtained by confocal microscopy, show the actin fibers<br />
that form the cytoskeleton.<br />
SaOs-2, a human osteosarcoma cell line, has been used as<br />
an osteoblast model. Cells were grown for 5 days on both<br />
structured <strong>and</strong> flat silicon surfaces. Cells on flat surfaces<br />
showed a good level of proliferation <strong>and</strong> a low level of<br />
apoptosis (also known as “programmed cell death”). Cells<br />
grown on short pillars had a similar behaviour. However, when<br />
the pillar height increased above 500 nm, the situation<br />
changed, with slow cell proliferation <strong>and</strong> increased apoptosis<br />
(Figure 2). Thus, it is possible to regulate the delicate<br />
equilibrium between proliferation <strong>and</strong> death by changing a<br />
single topographical parameter.<br />
In addition, the osteoblast morphology was also influenced by<br />
the change in pillar height [ 2 ] . In vitro studies have<br />
demonstrated that osteoblasts usually assume a flattened<br />
morphology on flat plastic surfaces. This flat form is very<br />
different from their in vivo morphology <strong>and</strong> represents a<br />
limitation of normal in vitro cell studies. In contrast, a more 3D<br />
morphology, similar to their physiological form was observed<br />
in samples on silicon surfaces with pillars over 500 nm high.<br />
This morphology is the result of internal rearrangements<br />
involving both the cytoskeleton [3] <strong>and</strong> the cell membrane <strong>and</strong><br />
which result in the disruption of focal adhesions culminating in<br />
a reduced cell-substrate adhesion.<br />
In conclusion, these silicon surfaces offer a way to regulate<br />
osteoblast behaviour in an in vitro system. Not only<br />
proliferation <strong>and</strong> apoptosis but also morphology are strongly<br />
modified. Cell behaviour on these surfaces may be closer to<br />
physiological (in vivo) behaviour, allowing in vitro studies of<br />
osteoblast that are more relevant <strong>and</strong> useful. Future work will<br />
include the study of bone cell differentiation <strong>and</strong> the<br />
production of fibrous <strong>and</strong> amorphous matrices in order to<br />
underst<strong>and</strong> whether osteoblasts on these substrates maintain<br />
their capacity to create bone.<br />
[1] J. M. Rice, J. A. Hunt, J. A. Gallagher, P. Hanarp,<br />
D. S. Sutherl<strong>and</strong>, “Quantitative assessment of the response of<br />
primary derived human osteoblasts <strong>and</strong> macrophages to a range<br />
of nanotopography surfaces in a single culture model in vitro”,<br />
Biomaterials, 24 (2003), 4799<br />
[2] Y. Wan, Y. Wang, Z. Liu, X. Qu, B. Han, J. Bei, S. Wang,<br />
“Adhesion <strong>and</strong> proliferation of OCT-1 osteoblast-like cells on<br />
micro- <strong>and</strong> nano-scale topography structured poly (L-lactide)”,<br />
Biomaterials, 46 (2005) 4453<br />
[3] H. M. Powell, D. A. Kniss, J. L. Lannutti, “Nanotopographic<br />
control of cytoskeletal organization”, Langmuir, 22 (2006), 5087<br />
59