CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
CSEM Scientific and Technical Report 2008
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
A Universal Protein Assembler<br />
H. Zepik<br />
Chemical protein synthesis: a new assembler molecule for the ligation of appropriately functionalized peptides in solution. The same novel<br />
approach can also be used for the purification of proteins, which is a very relevant problem today in the pharmaceutical industry<br />
Many powerful drugs are protein-based. Examples include<br />
hormones like insulin, interferons, monoclonal antibodies,<br />
blood clotting factors. Proteins are chains of amino acids, from<br />
a few tens to several hundreds. In recent years active<br />
pharmaceutical ingredients (APIs) based on proteins have<br />
received increased attention from the pharmaceutical industry.<br />
Peptide <strong>and</strong> protein therapeutics are attractive due to their<br />
high specificity <strong>and</strong> potency <strong>and</strong> low incidence of toxicology.<br />
In contrast to small molecule drugs, protein drugs are rarely<br />
produced by chemical synthesis. The major production<br />
methods are extraction from natural sources <strong>and</strong> recombinant<br />
DNA technology. However, chemical protein synthesis offers<br />
several advantages. It is possible to modify the chain of amino<br />
acids basically at-will, e.g. to insert unnatural amino acids with<br />
specific properties, to introduce various labels, to attach<br />
polymer- <strong>and</strong> sugar-chains. In addition the final product will<br />
not be contaminated by any undesired biological material.<br />
The chemical synthesis of peptide, has made enormous<br />
progress in the last decades <strong>and</strong> it is now possible to make<br />
peptides of up to ~50 amino acids reliably <strong>and</strong> in good yield.<br />
The accumulation of side products <strong>and</strong> aggregation prevent<br />
the synthesis of longer peptides <strong>and</strong> proteins. However, most<br />
protein drugs, like monoclonal antibodies, have more than<br />
100 amino acids.<br />
To overcome this size limit, peptide segments can be ligated<br />
in solution (Figure 1). However, due to the many reactive side<br />
chains present in proteins, exclusive formation of the desired<br />
bond only is impossible to achieve, unless a chemoselective<br />
condensation is employed. This last approach has been<br />
pioneered successfully but is restricted to certain amino acids<br />
due to the chemistry.<br />
NH 3 +<br />
NH 3 +<br />
SH<br />
SH<br />
NH 3 +<br />
NH 3 +<br />
OH<br />
OH<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
O<br />
C<br />
Figure 1: Unprotected peptide segments in solution cannot be ligated<br />
efficiently due to many reactive side chains.<br />
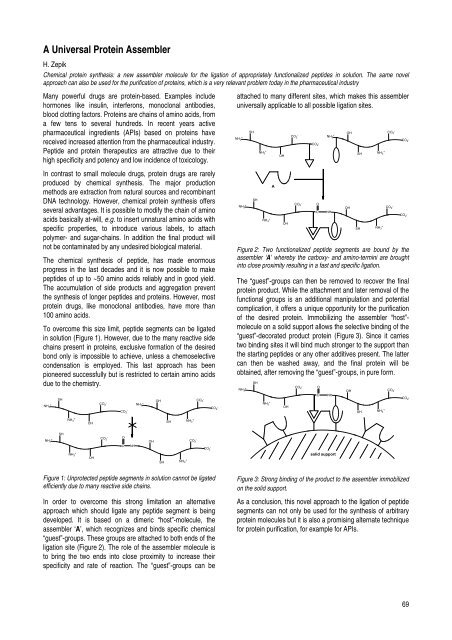
In order to overcome this strong limitation an alternative<br />
approach which should ligate any peptide segment is being<br />
developed. It is based on a dimeric “host”-molecule, the<br />
assembler ‘A’, which recognizes <strong>and</strong> binds specific chemical<br />
“guest”-groups. These groups are attached to both ends of the<br />
ligation site (Figure 2). The role of the assembler molecule is<br />
to bring the two ends into close proximity to increase their<br />
specificity <strong>and</strong> rate of reaction. The “guest”-groups can be<br />
NH<br />
NH 3 +<br />
OH<br />
OH<br />
SH<br />
SH<br />
NH 3 +<br />
NH 3 +<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
attached to many different sites, which makes this assembler<br />
universally applicable to all possible ligation sites.<br />
NH 3 +<br />
NH 3 +<br />
Figure 2: Two functionalized peptide segments are bound by the<br />
assembler ‘A’ whereby the carboxy- <strong>and</strong> amino-termini are brought<br />
into close proximity resulting in a fast <strong>and</strong> specific ligation.<br />
The “guest”-groups can then be removed to recover the final<br />
protein product. While the attachment <strong>and</strong> later removal of the<br />
functional groups is an additional manipulation <strong>and</strong> potential<br />
complication, it offers a unique opportunity for the purification<br />
of the desired protein. Immobilizing the assembler “host”molecule<br />
on a solid support allows the selective binding of the<br />
“guest”-decorated product protein (Figure 3). Since it carries<br />
two binding sites it will bind much stronger to the support than<br />
the starting peptides or any other additives present. The latter<br />
can then be washed away, <strong>and</strong> the final protein will be<br />
obtained, after removing the “guest”-groups, in pure form.<br />
NH 3 +<br />
SH<br />
SH<br />
SH<br />
NH 3 +<br />
NH 3 +<br />
NH 3 +<br />
A<br />
OH<br />
OH<br />
OH<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
O<br />
O<br />
C NH<br />
solid support<br />
Figure 3: Strong binding of the product to the assembler immobilized<br />
on the solid support.<br />
As a conclusion, this novel approach to the ligation of peptide<br />
segments can not only be used for the synthesis of arbitrary<br />
protein molecules but it is also a promising alternate technique<br />
for protein purification, for example for APIs.<br />
NH 3 +<br />
C NH<br />
OH<br />
OH<br />
OH<br />
SH<br />
SH<br />
SH<br />
NH 3 +<br />
NH 3 +<br />
NH 3 +<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
CO 2 -<br />
69