Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
H 3 CO<br />
H 3 CO<br />
H 3 CO<br />
NC C 2 H 5 H2 /PtO<br />
NH 2<br />
O O<br />
C 2 H 5 O OC 2 H 5<br />
H 3 CO<br />
C 2 H 5 OOC<br />
HN<br />
C 2 H 5<br />
COOC 2 H 5<br />
H 3 CO<br />
H 3 CO<br />
N<br />
C 2 H 5<br />
POCl 3<br />
Adams Catalyst<br />
H 3 CO<br />
H 3 CO<br />
O<br />
N<br />
COOC 2 H 5<br />
C 2 H 5<br />
1.<br />
H 3 CO<br />
H 3 CO<br />
NH 2 2.POCl 3<br />
3.H 2 /PtO<br />
COOC 2 H 5<br />
H 3 CO<br />
H 3 CO<br />
H 3 CO<br />
N<br />
C 2 H 5<br />
CH 2<br />
NH<br />
H 3 CO<br />
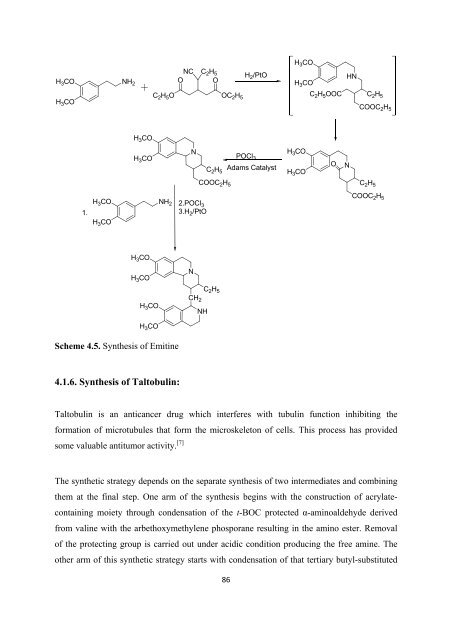
Scheme 4.5. Syn<strong>the</strong>sis <strong>of</strong> Emitine<br />
4.1.6. Syn<strong>the</strong>sis <strong>of</strong> Taltobulin:<br />
Taltobulin is an anticancer drug which interferes with tubulin function inhibiting <strong>the</strong><br />
<strong>for</strong>mation <strong>of</strong> microtubules that <strong>for</strong>m <strong>the</strong> microskeleton <strong>of</strong> cells. This process has provided<br />
some valuable antitumor activity. [7]<br />
The syn<strong>the</strong>tic strategy depends on <strong>the</strong> separate syn<strong>the</strong>sis <strong>of</strong> two intermediates and combining<br />
<strong>the</strong>m at <strong>the</strong> final step. One arm <strong>of</strong> <strong>the</strong> syn<strong>the</strong>sis begins with <strong>the</strong> construction <strong>of</strong> acrylatecontaining<br />
moiety through condensation <strong>of</strong> <strong>the</strong> t-BOC protected α-aminoaldehyde derived<br />
from valine with <strong>the</strong> arbethoxymethylene phosporane resulting in <strong>the</strong> amino ester. Removal<br />
<strong>of</strong> <strong>the</strong> protecting group is carried out under acidic condition producing <strong>the</strong> free amine. The<br />
o<strong>the</strong>r arm <strong>of</strong> this syn<strong>the</strong>tic strategy starts with condensation <strong>of</strong> that tertiary butyl-substituted<br />
86