Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
<strong>of</strong> imine reduction in <strong>the</strong> past eight years. Of course in <strong>the</strong> nineties great achievements were<br />
accomplished <strong>for</strong> complete picture please refer to <strong>the</strong> following review. [2]<br />
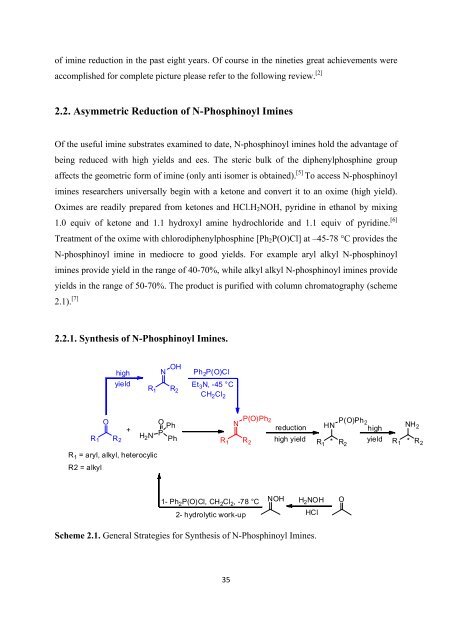
2.2. Asymmetric Reduction <strong>of</strong> N-Phosphinoyl Imines<br />
Of <strong>the</strong> useful imine substrates examined to date, N-phosphinoyl imines hold <strong>the</strong> advantage <strong>of</strong><br />
being reduced with high yields and ees. The steric bulk <strong>of</strong> <strong>the</strong> diphenylphosphine group<br />
affects <strong>the</strong> geometric <strong>for</strong>m <strong>of</strong> imine (only anti isomer is obtained). [5] To access N-phosphinoyl<br />
imines researchers universally begin with a ketone and convert it to an oxime (high yield).<br />
Oximes are readily prepared from ketones and HCl.H 2 NOH, pyridine in ethanol by mixing<br />
1.0 equiv <strong>of</strong> ketone and 1.1 hydroxyl amine hydrochloride and 1.1 equiv <strong>of</strong> pyridine. [6]<br />
Treatment <strong>of</strong> <strong>the</strong> oxime with chlorodiphenylphosphine [Ph 2 P(O)Cl] at –45-78 °C provides <strong>the</strong><br />
N-phosphinoyl imine in mediocre to good yields. For example aryl alkyl N-phosphinoyl<br />
imines provide yield in <strong>the</strong> range <strong>of</strong> 40-70%, while alkyl alkyl N-phosphinoyl imines provide<br />
yields in <strong>the</strong> range <strong>of</strong> 50-70%. The product is purified with column chromatography (scheme<br />
2.1). [7]<br />
2.2.1. Syn<strong>the</strong>sis <strong>of</strong> N-Phosphinoyl Imines.<br />
high<br />
yield<br />
N<br />
OH<br />
R 1 R 2<br />
Ph 2 P(O)Cl<br />
Et 3 N, -45 °C<br />
O<br />
R 1 R 2<br />
+<br />
H 2 N<br />
O<br />
P Ph<br />
Ph<br />
N<br />
R 1 R 2<br />
CH 2 Cl 2<br />
P(O)Ph 2 P(O)Ph2<br />
reduction<br />
high yield<br />
HN<br />
R 1<br />
* R 2<br />
high<br />
NH 2<br />
yield R 1 * R 2<br />
R 1 = aryl, alkyl, heterocylic<br />
R2 = alkyl<br />
1- Ph 2 P(O)Cl, CH 2 Cl 2 ,-78°C<br />
2- hydrolytic work-up<br />
NOH<br />
H 2 NOH<br />
HCl<br />
O<br />
Scheme 2.1. General Strategies <strong>for</strong> Syn<strong>the</strong>sis <strong>of</strong> N-Phosphinoyl Imines.<br />
35