Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
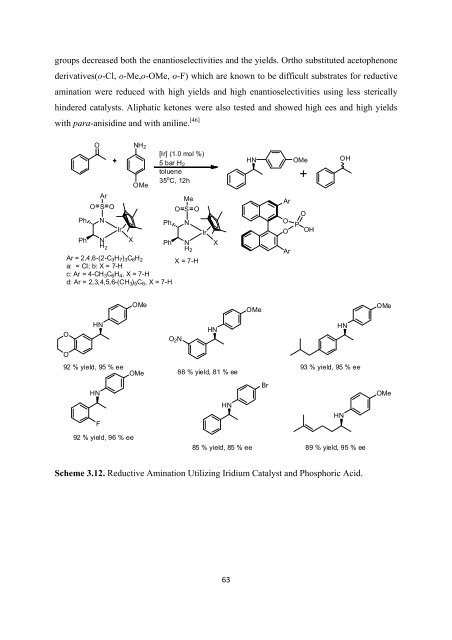
groups decreased both <strong>the</strong> enantioselectivities and <strong>the</strong> yields. Ortho substituted acetophenone<br />
derivatives(o-Cl, o-Me,o-OMe, o-F) which are known to be difficult substrates <strong>for</strong> reductive<br />
amination were reduced with high yields and high enantioselectivities using less sterically<br />
hindered catalysts. Aliphatic ketones were also tested and showed high ees and high yields<br />
with para-anisidine and with aniline. [46]<br />
Ph<br />
Ph<br />
O<br />
O NH 2<br />
Ar<br />
S<br />
N<br />
N<br />
H 2<br />
O<br />
Ir<br />
X<br />
OMe<br />
[Ir] (1.0 mol %)<br />
5barH 2<br />
toluene<br />
35 o C, 12h<br />
Ph<br />
Ph<br />
Ar = 2,4,6-(2-C 3 H 7 ) 3 C 6 H 2<br />
a: = Cl; b: X = 7-H<br />
c: Ar = 4-CH 3 C 6 H 4 ,X=7-H<br />
d: Ar = 2,3,4,5,6-(CH 3 ) 5 C 6 ,X=7-H<br />
O<br />
Me<br />
S<br />
N<br />
N<br />
H 2<br />
O<br />
X=7-H<br />
Ir<br />
X<br />
HN OMe OH<br />
Ar<br />
O<br />
O<br />
O<br />
P<br />
OH<br />
Ar<br />
OMe<br />
OMe<br />
OMe<br />
O<br />
HN<br />
O 2 N<br />
HN<br />
HN<br />
O<br />
92 % yield, 95 % ee<br />
OMe<br />
88 % yield, 81 % ee<br />
93 % yield, 95 % ee<br />
HN<br />
Br<br />
OMe<br />
HN<br />
F<br />
HN<br />
92 % yield, 96 % ee<br />
85 % yield, 85 % ee<br />
89 % yield, 95 % ee<br />
Scheme 3.12. Reductive Amination Utilizing Iridium Catalyst and Phosphoric Acid.<br />
63