Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O<br />
O<br />
O<br />
*<br />
NH<br />
Thalidomide<br />
(R)-active agent<br />
(S)-teratogenic<br />
O<br />
*<br />
H<br />
N<br />
OH<br />
Ethambutol<br />
(R,R)-blinding agent<br />
(S,S)-tuberculostatic<br />
2<br />
Limonene<br />
(S)-lemon odor<br />
Limonen<br />
(R)-organge odor<br />
O<br />
Carvone<br />
(S)-caraway<br />
O<br />
Carvone<br />
(R)-spearmint<br />
H 2 N<br />
OH 2 N<br />
H<br />
O<br />
Asparagine<br />
(S)-bitter<br />
OH<br />
HO<br />
O<br />
H<br />
NH 2 O<br />
Asparagine<br />
(R)-sweet<br />
NH 2<br />
HO<br />
H<br />
O<br />
O<br />
H<br />
N<br />
NH<br />
H 2<br />
O<br />
O<br />
O<br />
O<br />
H<br />
O<br />
O<br />
N<br />
H<br />
H 2 N<br />
H<br />
OH<br />
Aspartame<br />
(S,S)-sweet<br />
Aspartame<br />
(R,R)-bitter<br />
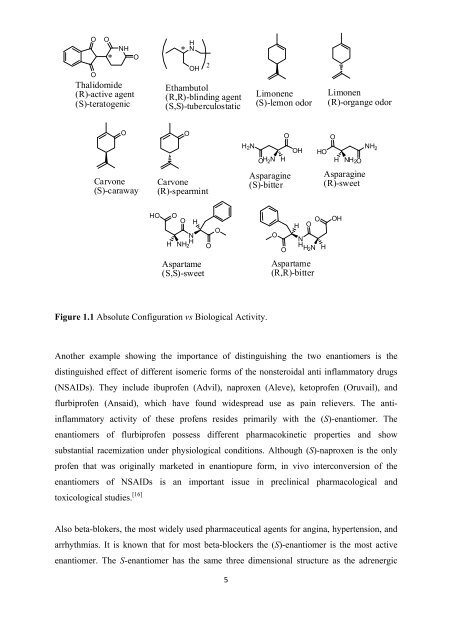
Figure 1.1 Absolute Configuration vs Biological Activity.<br />
Ano<strong>the</strong>r example showing <strong>the</strong> importance <strong>of</strong> distinguishing <strong>the</strong> two enantiomers is <strong>the</strong><br />
distinguished effect <strong>of</strong> different isomeric <strong>for</strong>ms <strong>of</strong> <strong>the</strong> nonsteroidal anti inflammatory drugs<br />
(NSAIDs). They include ibupr<strong>of</strong>en (Advil), naproxen (Aleve), ketopr<strong>of</strong>en (Oruvail), and<br />
flurbipr<strong>of</strong>en (Ansaid), which have found widespread use as pain relievers. The antiinflammatory<br />
activity <strong>of</strong> <strong>the</strong>se pr<strong>of</strong>ens resides primarily with <strong>the</strong> (S)-enantiomer. The<br />
enantiomers <strong>of</strong> flurbipr<strong>of</strong>en possess different pharmacokinetic properties and show<br />
substantial racemization under physiological conditions. Although (S)-naproxen is <strong>the</strong> only<br />
pr<strong>of</strong>en that was originally marketed in enantiopure <strong>for</strong>m, in vivo interconversion <strong>of</strong> <strong>the</strong><br />
enantiomers <strong>of</strong> NSAIDs is an important issue in preclinical pharmacological and<br />
toxicological studies. [16]<br />
Also beta-blokers, <strong>the</strong> most widely used pharmaceutical agents <strong>for</strong> angina, hypertension, and<br />
arrhythmias. It is known that <strong>for</strong> most beta-blockers <strong>the</strong> (S)-enantiomer is <strong>the</strong> most active<br />
enantiomer. The S-enantiomer has <strong>the</strong> same three dimensional structure as <strong>the</strong> adrenergic<br />
5