Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
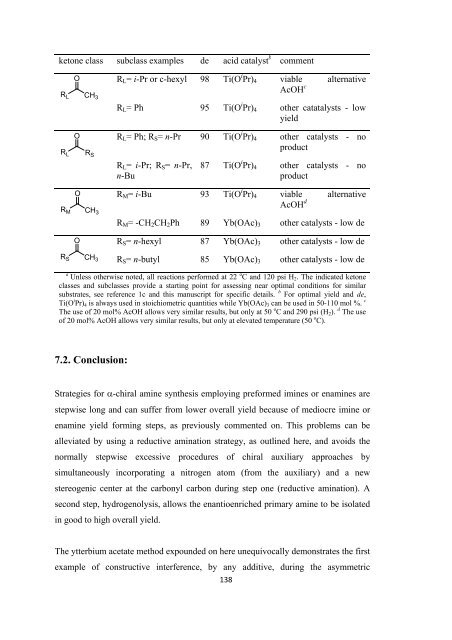
ketone class subclass examples de acid catalyst b comment<br />
O R L = i-Pr or c-hexyl 98 Ti(O i Pr) 4 viable alternative<br />
AcOH c<br />
R L CH 3<br />
R L = Ph 95 Ti(O i Pr) 4 o<strong>the</strong>r catatalysts - low<br />
yield<br />
R L<br />
O R L = Ph; R S = n-Pr 90 Ti(O i Pr) 4 o<strong>the</strong>r catalysts - no<br />
product<br />
R S<br />
R L = i-Pr; R S = n-Pr,<br />
n-Bu<br />
87 Ti(O i Pr) 4 o<strong>the</strong>r catalysts - no<br />
product<br />
O R M = i-Bu 93 Ti(O i Pr) 4 viable alternative<br />
AcOH d<br />
R M CH 3<br />
R M = -CH 2 CH 2 Ph 89 Yb(OAc) 3 o<strong>the</strong>r catalysts - low de<br />
O R S = n-hexyl 87 Yb(OAc) 3 o<strong>the</strong>r catalysts - low de<br />
R S CH 3<br />
R S = n-butyl 85 Yb(OAc) 3 o<strong>the</strong>r catalysts - low de<br />
a<br />
Unless o<strong>the</strong>rwise noted, all reactions per<strong>for</strong>med at 22 o C and 120 psi H 2 . The indicated ketone<br />
classes and subclasses provide a starting point <strong>for</strong> assessing near optimal conditions <strong>for</strong> similar<br />
substrates, see reference 1c and this manuscript <strong>for</strong> specific details. b For optimal yield and de,<br />
Ti(O i Pr) 4 is always used in stoichiometric quantities while Yb(OAc) 3 can be used in 50-110 mol %. c<br />
The use <strong>of</strong> 20 mol% AcOH allows very similar results, but only at 50 o C and 290 psi (H 2 ). d The use<br />
<strong>of</strong> 20 mol% AcOH allows very similar results, but only at elevated temperature (50 o C).<br />
7.2. Conclusion:<br />
Strategies <strong>for</strong> α-chiral amine syn<strong>the</strong>sis employing pre<strong>for</strong>med imines or enamines are<br />
stepwise long and can suffer from lower overall yield because <strong>of</strong> mediocre imine or<br />
enamine yield <strong>for</strong>ming steps, as previously commented on. This problems can be<br />
alleviated by using a reductive amination strategy, as outlined here, and avoids <strong>the</strong><br />
normally stepwise excessive procedures <strong>of</strong> chiral auxiliary approaches by<br />
simultaneously incorporating a nitrogen atom (from <strong>the</strong> auxiliary) and a new<br />
stereogenic center at <strong>the</strong> carbonyl carbon during step one (reductive amination). A<br />
second step, hydrogenolysis, allows <strong>the</strong> enantioenriched primary amine to be isolated<br />
in good to high overall yield.<br />
The ytterbium acetate method expounded on here unequivocally demonstrates <strong>the</strong> first<br />
example <strong>of</strong> constructive interference, by any additive, during <strong>the</strong> asymmetric<br />
138