- Page 1 and 2: Improved Methodology for the Prepar
- Page 3 and 4: This dissertation is dedicated to a
- Page 5 and 6: suppressing alcohol formation and p
- Page 7 and 8: Prof. Mohamed El-Azizi, Prof. Abdel
- Page 9 and 10: Et EtOH EtOAc GC h HPLC HRMS Hz J K
- Page 11 and 12: Table of Contents Abstract. Acknowl
- Page 13 and 14: 4.1.5. Synthesis of Emitine 85 4.1.
- Page 15 and 16: Chapter 1 Introduction 1.1. Chiral
- Page 17 and 18: 1. Cis-trans or geometric isomers.
- Page 19 and 20: O O O * NH Thalidomide (R)-active a
- Page 21 and 22: One enantiomer may be responsible f
- Page 23 and 24: 1.5.1. Synthesis of Enantiomericall
- Page 25 and 26: 1.5.2.2. Kinetic Resolution Kinetic
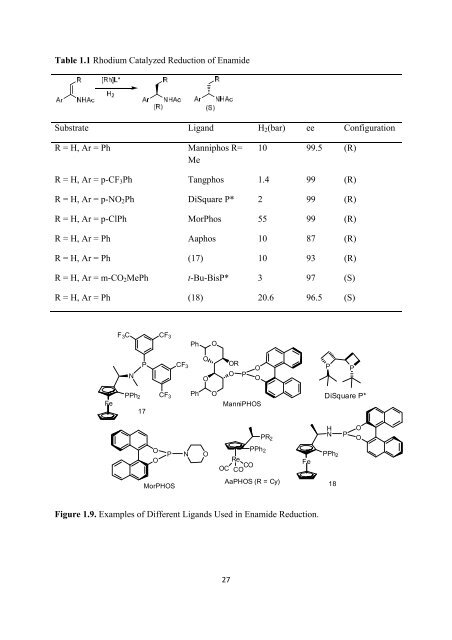
- Page 27 and 28: compound by the auxiliary. The auxi
- Page 29 and 30: 1.5.4. Stereoselective Conversion o
- Page 31 and 32: It is estimated that 3000 tonnes (a
- Page 33 and 34: k R R P 1 k rac S k S P 2 Figure 1.
- Page 35 and 36: (S)-(α)-Methylbenzylamine and its
- Page 37 and 38: fourth chapter showing different dr
- Page 39: Burk was successful in reducing ary
- Page 43 and 44: [8] E. L. Eliel, S. H. Wilen, Stere
- Page 45 and 46: [42] a) J. Blacker, Innovations in
- Page 47 and 48: [67] a) H. Qin, N. Yamagiwa, S. Mat
- Page 49 and 50: of imine reduction in the past eigh
- Page 51 and 52: 8 years beginning from the year 200
- Page 53 and 54: 2.2.3. Nguyen Special Substrates. A
- Page 55 and 56: Figure 2.4 General Catalyst structu
- Page 57 and 58: 2.3.2. Different Substrates Categor
- Page 59 and 60: H 2 , toluene, 25 °C, 4 h an ee of
- Page 61 and 62: 1). They described the role of each
- Page 63 and 64: More recently, 2008, he described t
- Page 65 and 66: [22] E. Guiu, M. Aghmiz, Y. Diaz, C
- Page 67 and 68: Chapter 3 Reductive Amination 3.1.
- Page 69 and 70: . CH 3 CO(CH 2 ) 5 CH 3 H 2 NCH 2 C
- Page 71 and 72: superior in terms of conversion (89
- Page 73 and 74: O NHR 3 R 4 R 4 R 3 N OTi(O i Pr) 3
- Page 75 and 76: Scheme 3.9. Synthesis of (S)-Metola
- Page 77 and 78: groups decreased both the enantiose
- Page 79 and 80: progress of the reaction was monito
- Page 81 and 82: attempts were directed for the asym
- Page 83 and 84: hydrogen bonding, is chiral and its
- Page 85 and 86: Later he utilized this methodology
- Page 87 and 88: OMe OMe O HN HN HN O 2 N 71 % yield
- Page 89 and 90: compared to the oil refinery indust
- Page 91 and 92:
[14] V. I. Tararov, R. Kadyrov, T.H
- Page 93 and 94:
[55] a) R. Kadyrov, T. H. Riermeier
- Page 95 and 96:
Chapter 4 Drugs and Reductive Amina
- Page 97 and 98:
Scheme 4.2. Synthesis of Muraglitaz
- Page 99 and 100:
Studies showed that the active isom
- Page 101 and 102:
aminoacid producing the protected a
- Page 103 and 104:
O NH 2 1. p-TsOH/toluene 2. BH 3 -T
- Page 105 and 106:
O O 125 NH 2 a 93% O O 126 H Bu N T
- Page 107 and 108:
ethyl carbamate by acylation with e
- Page 109 and 110:
4.1.16. Synthesis of Ritonavir and
- Page 111 and 112:
4.2. Conclusion Different important
- Page 113 and 114:
Chapter 5 Stoichiometric Use of Ytt
- Page 115 and 116:
The unique feature of this methodol
- Page 117 and 118:
Ytterbium triflate is the most comm
- Page 119 and 120:
Ruthenium(III) chloride 31.7 4 4.2
- Page 121 and 122:
t-Butyl methyl ether 15 - - 82 Hexa
- Page 123 and 124:
2-octanone starting material. When
- Page 125 and 126:
3 Yb(OTf) 3 d 4 Ti(O i Pr) 4 e 5 B(
- Page 127 and 128:
If a [1,3]-proton shift of the init
- Page 129 and 130:
enhanced stereoselectivity. For exa
- Page 131 and 132:
WO2006030017, 2006; c) T. C. Nugent
- Page 133 and 134:
4 60 86 5 50 86 6 40 84 7 20 79 8 2
- Page 135 and 136:
The reactions described above all u
- Page 137 and 138:
e.g. compare entries 1, 5, 6, and 9
- Page 139 and 140:
solvent in the stoichiometric and c
- Page 141 and 142:
[2] Farina, V.; Grozinger, K.; Mül
- Page 143 and 144:
congested which should be favored.
- Page 145 and 146:
imine area % (GC analysis) time (mi
- Page 147 and 148:
Inversion at the nitrogen atom of t
- Page 149 and 150:
anti-6) would be expected to have m
- Page 151 and 152:
e.g. AcOH, suppresses alcohol by-pr
- Page 153 and 154:
eductive amination of a prochiral k
- Page 155 and 156:
Appendix Experimental Section Gener
- Page 157 and 158:
and the mixture was stirred for 30
- Page 159 and 160:
Reaction details: Yb(OAc) 3 (1.1 eq
- Page 161 and 162:
etention time [min]: major (S,S)-2b
- Page 163 and 164:
time [min]: major (S,S)-2c isomer,
- Page 165 and 166:
obtain the hydrochloride salt (0.41
- Page 167 and 168:
with etheral HCl provided the hydro
- Page 169 and 170:
Research experience: Date Project S