Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
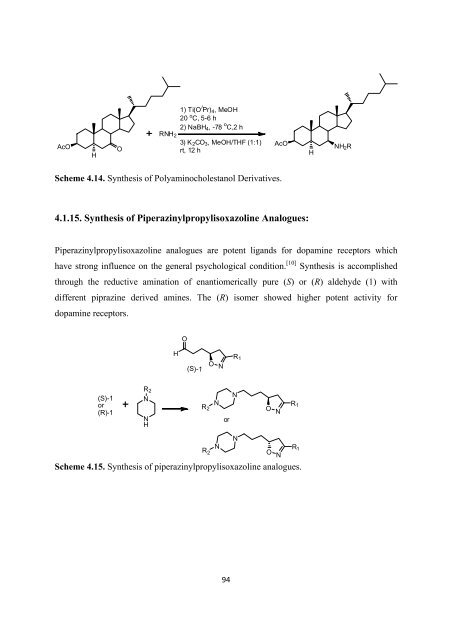
AcO<br />
H<br />
O<br />
1) Ti(O i Pr) 4 ,MeOH<br />
20 o C, 5-6 h<br />
2) NaBH 4 ,-78 o C,2 h<br />
RNH 2<br />
3) K 2 CO 3 , MeOH/THF (1:1)<br />
rt, 12 h<br />
AcO<br />
H<br />
NH 2 R<br />
Scheme 4.14. Syn<strong>the</strong>sis <strong>of</strong> Polyaminocholestanol Derivatives.<br />
4.1.15. Syn<strong>the</strong>sis <strong>of</strong> Piperazinylpropylisoxazoline Analogues:<br />
Piperazinylpropylisoxazoline analogues are potent ligands <strong>for</strong> dopamine receptors which<br />
have strong influence on <strong>the</strong> general psychological condition. [10] Syn<strong>the</strong>sis is accomplished<br />
through <strong>the</strong> reductive amination <strong>of</strong> enantiomerically pure (S) or (R) aldehyde (1) with<br />
different piprazine derived amines. The (R) isomer showed higher potent activity <strong>for</strong><br />
dopamine receptors.<br />
O<br />
H<br />
(S)-1<br />
O N<br />
R 1<br />
(S)-1<br />
or<br />
(R)-1<br />
N<br />
N<br />
N<br />
O N<br />
R 1<br />
R 2<br />
N<br />
H<br />
or<br />
R 2<br />
O N<br />
R 1<br />
R 2<br />
N<br />
N<br />
Scheme 4.15. Syn<strong>the</strong>sis <strong>of</strong> piperazinylpropylisoxazoline analogues.<br />
94