Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
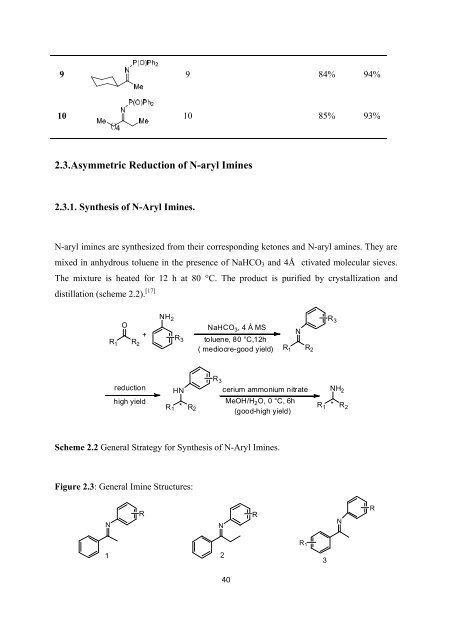
9 9 84% 94%<br />
10 10 85% 93%<br />
2.3.Asymmetric Reduction <strong>of</strong> N-aryl Imines<br />
2.3.1. Syn<strong>the</strong>sis <strong>of</strong> N-Aryl Imines.<br />
N-aryl imines are syn<strong>the</strong>sized from <strong>the</strong>ir corresponding ketones and N-aryl amines. They are<br />
mixed in anhydrous toluene in <strong>the</strong> presence <strong>of</strong> NaHCO 3 and 4Å ctivated molecular sieves.<br />
The mixture is heated <strong>for</strong> 12 h at 80 °C. The product is purified by crystallization and<br />
distillation (scheme 2.2). [17]<br />
NH 2<br />
R 3<br />
O<br />
+<br />
R 1 R 2<br />
NaHCO 3 ,4ÅMS<br />
toluene, 80 °C,12h<br />
( mediocre-good yield)<br />
N<br />
R 1 R 2<br />
R 3<br />
reduction<br />
high yield<br />
R 3<br />
HN<br />
cerium ammonium nitrate NH 2<br />
MeOH/H 2 O, 0 °C, 6h<br />
R 1<br />
* R 2<br />
R 1<br />
* R 2<br />
(good-high yield)<br />
Scheme 2.2 General Strategy <strong>for</strong> Syn<strong>the</strong>sis <strong>of</strong> N-Aryl Imines.<br />
Figure 2.3: General Imine Structures:<br />
N<br />
R<br />
N<br />
R<br />
N<br />
R<br />
R 1<br />
1<br />
2<br />
3<br />
40