Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
1<br />
1g<br />
O<br />
2g HN Ph<br />
81<br />
98<br />
2<br />
1h<br />
O<br />
2h HN Ph<br />
78<br />
98<br />
3<br />
1i<br />
O<br />
2i HN Ph<br />
63<br />
94<br />
1b<br />
O<br />
2b<br />
HN<br />
Ph<br />
78<br />
92<br />
5<br />
4 d 92<br />
O<br />
1a<br />
HN<br />
Ph<br />
2a<br />
79<br />
6<br />
1c<br />
O<br />
2c<br />
HN<br />
Ph<br />
87<br />
80<br />
7<br />
1f<br />
O<br />
2f<br />
HN<br />
Ph<br />
82<br />
e<br />
79<br />
8<br />
1d<br />
O<br />
2d<br />
HN<br />
Ph<br />
83<br />
72<br />
9<br />
1e<br />
O<br />
2e<br />
HN<br />
Ph<br />
82<br />
71<br />
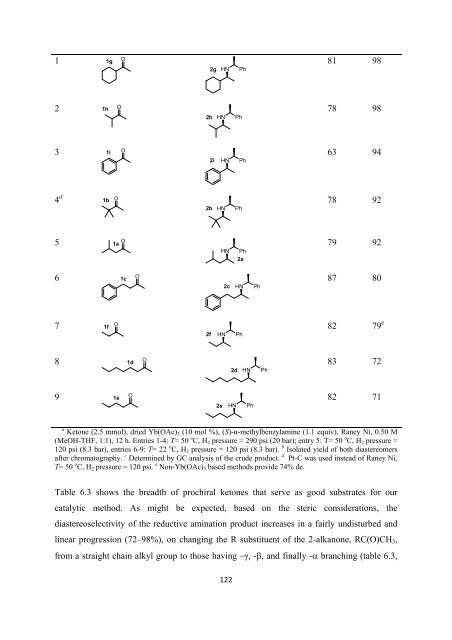
a Ketone (2.5 mmol), dried Yb(OAc) 3 (10 mol %), (S)-α-methylbenzylamine (1.1 equiv), Raney Ni, 0.50 M<br />
(MeOH-THF, 1:1), 12 h. Entries 1-4: T= 50 o C, H 2 pressure = 290 psi (20 bar); entry 5: T= 50 o C, H 2 pressure =<br />
120 psi (8.3 bar), entries 6-9: T= 22 o C, H 2 pressure = 120 psi (8.3 bar). b Isolated yield <strong>of</strong> both diastereomers<br />
after chromatography. c Determined by GC analysis <strong>of</strong> <strong>the</strong> crude product. d Pt-C was used instead <strong>of</strong> Raney Ni,<br />
T= 50 o C, H 2 pressure = 120 psi. e Non-Yb(OAc) 3 based methods provide 74% de.<br />
Table 6.3 shows <strong>the</strong> breadth <strong>of</strong> prochiral ketones that serve as good substrates <strong>for</strong> our<br />
catalytic method. As might be expected, based on <strong>the</strong> steric considerations, <strong>the</strong><br />
diastereoselectivity <strong>of</strong> <strong>the</strong> reductive amination product increases in a fairly undisturbed and<br />
linear progression (72–98%), on changing <strong>the</strong> R substituent <strong>of</strong> <strong>the</strong> 2-alkanone, RC(O)CH 3 ,<br />
from a straight chain alkyl group to those having –γ, -β, and finally -α branching (table 6.3,<br />
122