Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
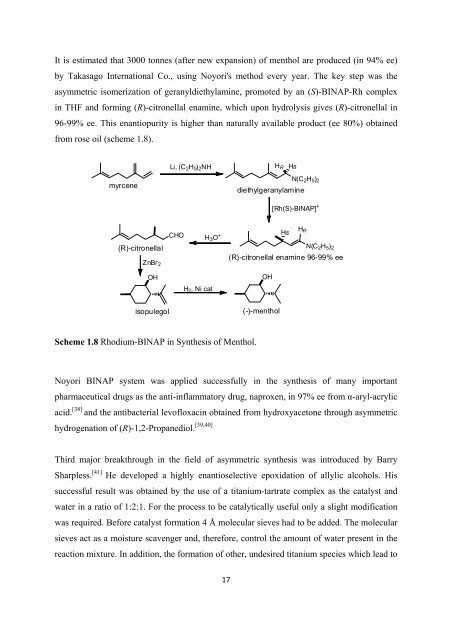
It is estimated that 3000 tonnes (after new expansion) <strong>of</strong> menthol are produced (in 94% ee)<br />
by Takasago International Co., using Noyori's method every year. The key step was <strong>the</strong><br />
asymmetric isomerization <strong>of</strong> geranyldiethylamine, promoted by an (S)-BINAP-Rh complex<br />
in THF and <strong>for</strong>ming (R)-citronellal enamine, which upon hydrolysis gives (R)-citronellal in<br />
96-99% ee. This enantiopurity is higher than naturally available product (ee 80%) obtained<br />
from rose oil (scheme 1.8).<br />
Li, (C 2 H 5 ) 2 NH<br />
H R<br />
Hs<br />
myrcene<br />
diethylgeranylamine<br />
N(C 2 H 5 ) 2<br />
[Rh(S)-BINAP] +<br />
CHO<br />
H 3 O +<br />
Hs<br />
H R<br />
(R)-citronellal<br />
ZnBr 2<br />
N(C 2 H 5 ) 2<br />
(R)-citronellal enamine 96-99% ee<br />
OH<br />
OH<br />
H 2 ,Nicat<br />
isopulegol<br />
(-)-menthol<br />
Scheme 1.8 Rhodium-BINAP in Syn<strong>the</strong>sis <strong>of</strong> Menthol.<br />
Noyori BINAP system was applied successfully in <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> many important<br />
pharmaceutical drugs as <strong>the</strong> anti-inflammatory drug, naproxen, in 97% ee from α-aryl-acrylic<br />
acid. [38] and <strong>the</strong> antibacterial lev<strong>of</strong>loxacin obtained from hydroxyacetone through asymmetric<br />
hydrogenation <strong>of</strong> (R)-1,2-Propanediol. [39,40]<br />
Third major breakthrough in <strong>the</strong> field <strong>of</strong> asymmetric syn<strong>the</strong>sis was introduced by Barry<br />
Sharpless. [41] He developed a highly enantioselective epoxidation <strong>of</strong> allylic alcohols. His<br />
successful result was obtained by <strong>the</strong> use <strong>of</strong> a titanium-tartrate complex as <strong>the</strong> catalyst and<br />
water in a ratio <strong>of</strong> 1:2:1. For <strong>the</strong> process to be catalytically useful only a slight modification<br />
was required. Be<strong>for</strong>e catalyst <strong>for</strong>mation 4 Å molecular sieves had to be added. The molecular<br />
sieves act as a moisture scavenger and, <strong>the</strong>re<strong>for</strong>e, control <strong>the</strong> amount <strong>of</strong> water present in <strong>the</strong><br />
reaction mixture. In addition, <strong>the</strong> <strong>for</strong>mation <strong>of</strong> o<strong>the</strong>r, undesired titanium species which lead to<br />
17