Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
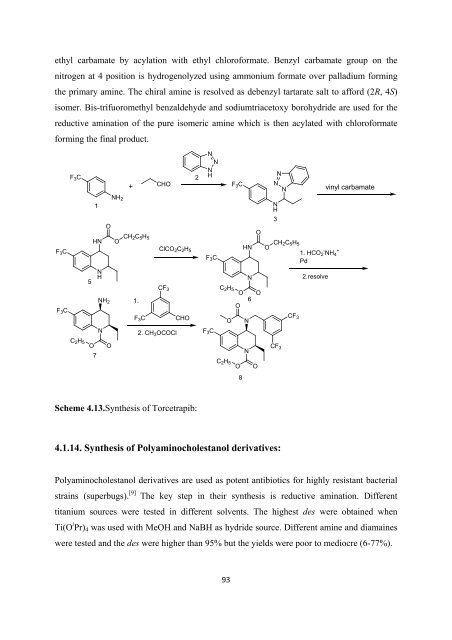
ethyl carbamate by acylation with ethyl chloro<strong>for</strong>mate. Benzyl carbamate group on <strong>the</strong><br />
nitrogen at 4 position is hydrogenolyzed using ammonium <strong>for</strong>mate over palladium <strong>for</strong>ming<br />
<strong>the</strong> primary amine. The chiral amine is resolved as debenzyl tartarate salt to af<strong>for</strong>d (2R, 4S)<br />
isomer. Bis-trifuoromethyl benzaldehyde and sodiumtriacetoxy borohydride are used <strong>for</strong> <strong>the</strong><br />
reductive amination <strong>of</strong> <strong>the</strong> pure isomeric amine which is <strong>the</strong>n acylated with chloro<strong>for</strong>mate<br />
<strong>for</strong>ming <strong>the</strong> final product.<br />
F 3 C<br />
F 3 C<br />
5<br />
1<br />
HN<br />
N<br />
H<br />
O<br />
NH 2<br />
O<br />
CH 2 C 5 H 5<br />
CHO<br />
ClCO 2 C 2 H 5<br />
CF 3<br />
NH 2 1.<br />
F 3 C<br />
F 3 C CHO<br />
N<br />
2. CH 3 OCOCl<br />
C 2 H 5<br />
O O<br />
7<br />
2<br />
N<br />
N<br />
N<br />
H<br />
F 3 C<br />
O<br />
N<br />
N<br />
N<br />
N<br />
H<br />
CH 2 C 5 H 5<br />
HN O<br />
1. HCO - +<br />
2 NH 4<br />
F 3 C<br />
Pd<br />
N<br />
2.resolve<br />
C 2 H 5<br />
O O<br />
6<br />
O<br />
F 3 C<br />
O<br />
C 2 H 5<br />
O<br />
8<br />
N<br />
N<br />
O<br />
3<br />
CF 3<br />
CF 3<br />
vinyl carbamate<br />
Scheme 4.13.Syn<strong>the</strong>sis <strong>of</strong> Torcetrapib:<br />
4.1.14. Syn<strong>the</strong>sis <strong>of</strong> Polyaminocholestanol derivatives:<br />
Polyaminocholestanol derivatives are used as potent antibiotics <strong>for</strong> highly resistant bacterial<br />
strains (superbugs). [9] The key step in <strong>the</strong>ir syn<strong>the</strong>sis is reductive amination. Different<br />
titanium sources were tested in different solvents. The highest des were obtained when<br />
Ti(O i Pr) 4 was used with MeOH and NaBH as hydride source. Different amine and diamaines<br />
were tested and <strong>the</strong> des were higher than 95% but <strong>the</strong> yields were poor to mediocre (6-77%).<br />
93