Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
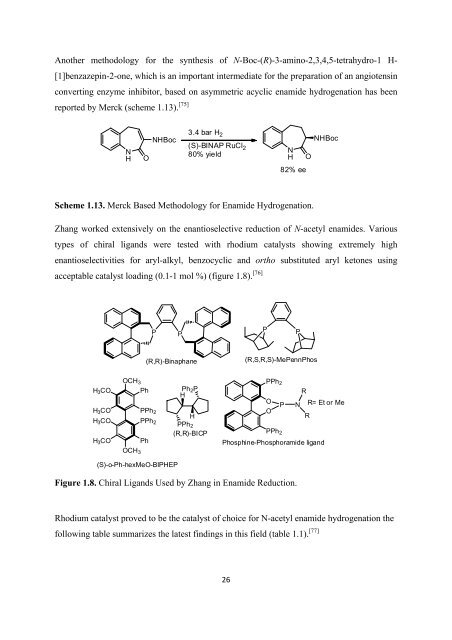
Ano<strong>the</strong>r methodology <strong>for</strong> <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> N-Boc-(R)-3-amino-2,3,4,5-tetrahydro-1 H-<br />
[1]benzazepin-2-one, which is an important intermediate <strong>for</strong> <strong>the</strong> preparation <strong>of</strong> an angiotensin<br />
converting enzyme inhibitor, based on asymmetric acyclic enamide hydrogenation has been<br />
reported by Merck (scheme 1.13). [75]<br />
N<br />
H<br />
O<br />
NHBoc<br />
3.4 bar H 2<br />
(S)-BINAP RuCl 2<br />
80% yield<br />
N<br />
H O<br />
82% ee<br />
NHBoc<br />
Scheme 1.13. Merck Based <strong>Methodology</strong> <strong>for</strong> Enamide Hydrogenation.<br />
Zhang worked extensively on <strong>the</strong> enantioselective reduction <strong>of</strong> N-acetyl enamides. Various<br />
types <strong>of</strong> chiral ligands were tested with rhodium catalysts showing extremely high<br />
enantioselectivities <strong>for</strong> aryl-alkyl, benzocyclic and ortho substituted aryl ketones using<br />
acceptable catalyst loading (0.1-1 mol %) (figure 1.8). [76]<br />
P<br />
P<br />
P<br />
P<br />
(R,R)-Binaphane<br />
(R,S,R,S)-MePennPhos<br />
OCH 3<br />
H 3 CO Ph<br />
H 3 CO PPh 2<br />
H 3 CO PPh 2<br />
H 3 CO Ph<br />
OCH 3<br />
Ph 2 P<br />
H<br />
H<br />
PPh 2<br />
(R,R)-BICP<br />
PPh 2<br />
O<br />
O<br />
PPh 2<br />
Phosphine-Phosphoramide ligand<br />
P<br />
R<br />
N R= Et or Me<br />
R<br />
(S)-o-Ph-hexMeO-BIPHEP<br />
Figure 1.8. <strong>Chiral</strong> Ligands Used by Zhang in Enamide Reduction.<br />
Rhodium catalyst proved to be <strong>the</strong> catalyst <strong>of</strong> choice <strong>for</strong> N-acetyl enamide hydrogenation <strong>the</strong><br />
following table summarizes <strong>the</strong> latest findings in this field (table 1.1). [77]<br />
26