Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
EtO 2 C<br />
H<br />
H<br />
CO 2 Et<br />
R<br />
O<br />
H<br />
+<br />
H 2 N<br />
OMe<br />
N<br />
H<br />
S<br />
(1.1 equiv)<br />
R<br />
N<br />
H<br />
OMe<br />
H 2 N NH 2<br />
(1.0 equiv)<br />
5 Å MS, toluene, 70 °C<br />
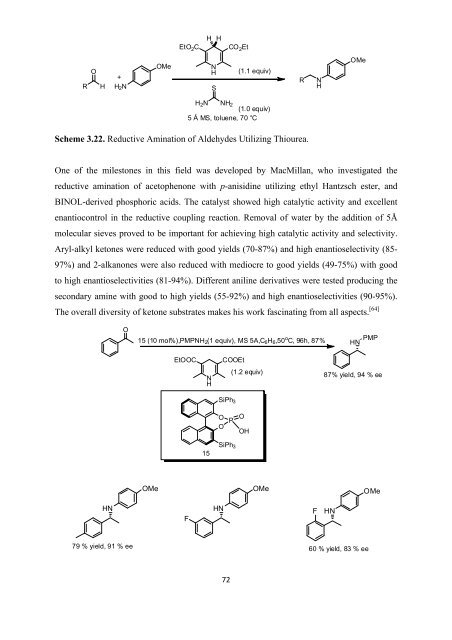
Scheme 3.22. Reductive Amination <strong>of</strong> Aldehydes Utilizing Thiourea.<br />
One <strong>of</strong> <strong>the</strong> milestones in this field was developed by MacMillan, who investigated <strong>the</strong><br />
reductive amination <strong>of</strong> acetophenone with p-anisidine utilizing ethyl Hantzsch ester, and<br />
BINOL-derived phosphoric acids. The catalyst showed high catalytic activity and excellent<br />
enantiocontrol in <strong>the</strong> reductive coupling reaction. Removal <strong>of</strong> water by <strong>the</strong> addition <strong>of</strong> 5Å<br />
molecular sieves proved to be important <strong>for</strong> achieving high catalytic activity and selectivity.<br />
Aryl-alkyl ketones were reduced with good yields (70-87%) and high enantioselectivity (85-<br />
97%) and 2-alkanones were also reduced with mediocre to good yields (49-75%) with good<br />
to high enantioselectivities (81-94%). Different aniline derivatives were tested producing <strong>the</strong><br />
secondary amine with good to high yields (55-92%) and high enantioselectivities (90-95%).<br />
The overall diversity <strong>of</strong> ketone substrates makes his work fascinating from all aspects. [64]<br />
O<br />
15 (10 mol%),PMPNH 2 (1 equiv), MS 5A,C 6 H 6 ,50 o C, 96h, 87%<br />
HN PMP<br />
EtOOC<br />
COOEt<br />
N<br />
H<br />
(1.2 equiv)<br />
87% yield, 94 % ee<br />
SiPh 3<br />
O<br />
O<br />
P<br />
O OH<br />
15<br />
SiPh 3<br />
OMe<br />
OMe<br />
OMe<br />
HN<br />
F<br />
HN<br />
F<br />
HN<br />
79 % yield, 91 % ee<br />
60 % yield, 83 % ee<br />
72