Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2-octanone starting material. When <strong>the</strong> binary solvent system <strong>of</strong> MeOH-THF (1:1) was<br />
examined, an 86% de was consistently achieved with a fast reaction time <strong>of</strong> 10-12 h. The<br />
same solvent systems which proved to be efficient in <strong>the</strong> reductive amination <strong>of</strong><br />
benzylacetone were also useful in <strong>the</strong> reductive amination <strong>of</strong> 2-octanone. From solvent<br />
screening studies it was obvious that <strong>the</strong> presence <strong>of</strong> MeOH in <strong>the</strong> solvent mixture is essential<br />
<strong>for</strong> <strong>the</strong> fast reaction rate. Replacement <strong>of</strong> THF in THF-MeOH mixture with o<strong>the</strong>r solvents<br />
resulted in lower de or/and prolonged reaction time. The same de was obtained through<br />
replacing THF in <strong>the</strong> THF-MeOH system with toluene, Et 2 O, or 1,3-dioxolane but with<br />
moderately longer reaction times. Replacing MeOH with EtOH in THF-MeOH system<br />
resulted in <strong>the</strong> same de but <strong>the</strong> reaction time was longer (24h). To ensure that this high<br />
diastereoselectivity is maintained and no racemization is occurring, I hydrogenolyzed <strong>the</strong><br />
reductive amination product to ensure that <strong>the</strong> enantiopurity <strong>of</strong> <strong>the</strong> primary amine is<br />
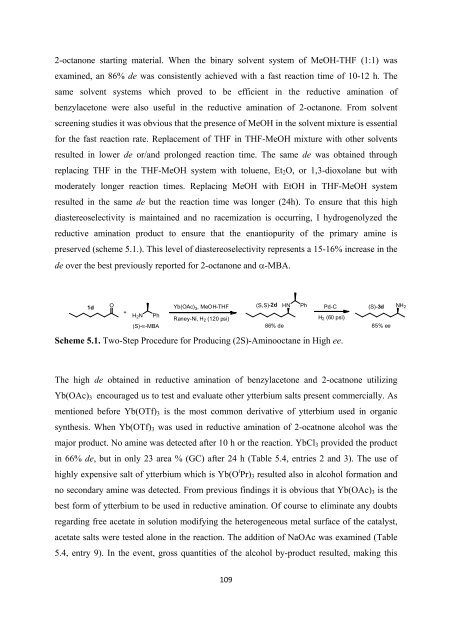
preserved (scheme 5.1.). This level <strong>of</strong> diastereoselectivity represents a 15-16% increase in <strong>the</strong><br />
de over <strong>the</strong> best previously reported <strong>for</strong> 2-octanone and α-MBA.<br />
1d<br />
O<br />
+<br />
H 2 N<br />
Ph<br />
(S)-α-MBA<br />
Yb(OAc)3 ,MeOH-THF<br />
Raney-Ni, H 2 (120 psi)<br />
(S,S)-2d HN Ph Pd-C<br />
(S)-3d NH 2<br />
H 2 (60 psi)<br />
86% de 85% ee<br />
Scheme 5.1. Two-Step Procedure <strong>for</strong> Producing (2S)-Aminooctane in High ee.<br />
The high de obtained in reductive amination <strong>of</strong> benzylacetone and 2-ocatnone utilizing<br />
Yb(OAc) 3 encouraged us to test and evaluate o<strong>the</strong>r ytterbium salts present commercially. As<br />
mentioned be<strong>for</strong>e Yb(OTf) 3 is <strong>the</strong> most common derivative <strong>of</strong> ytterbium used in organic<br />
syn<strong>the</strong>sis. When Yb(OTf) 3 was used in reductive amination <strong>of</strong> 2-ocatnone alcohol was <strong>the</strong><br />
major product. No amine was detected after 10 h or <strong>the</strong> reaction. YbCl 3 provided <strong>the</strong> product<br />
in 66% de, but in only 23 area % (GC) after 24 h (Table 5.4, entries 2 and 3). The use <strong>of</strong><br />
highly expensive salt <strong>of</strong> ytterbium which is Yb(O i Pr) 3 resulted also in alcohol <strong>for</strong>mation and<br />
no secondary amine was detected. From previous findings it is obvious that Yb(OAc) 3 is <strong>the</strong><br />
best <strong>for</strong>m <strong>of</strong> ytterbium to be used in reductive amination. Of course to eliminate any doubts<br />
regarding free acetate in solution modifying <strong>the</strong> heterogeneous metal surface <strong>of</strong> <strong>the</strong> catalyst,<br />
acetate salts were tested alone in <strong>the</strong> reaction. The addition <strong>of</strong> NaOAc was examined (Table<br />
5.4, entry 9). In <strong>the</strong> event, gross quantities <strong>of</strong> <strong>the</strong> alcohol by-product resulted, making this<br />
109