Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
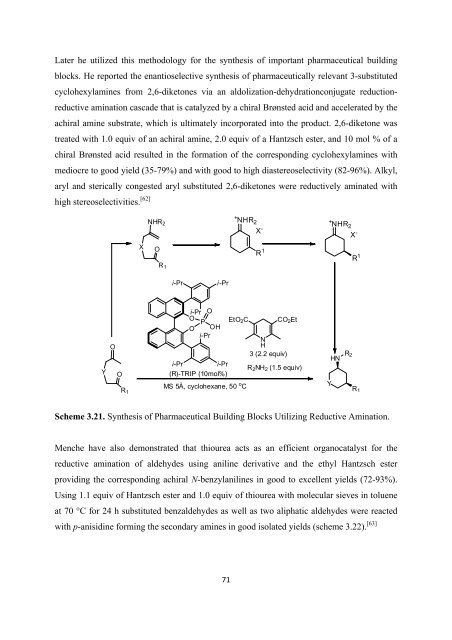
Later he utilized this methodology <strong>for</strong> <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> important pharmaceutical building<br />
blocks. He reported <strong>the</strong> enantioselective syn<strong>the</strong>sis <strong>of</strong> pharmaceutically relevant 3-substituted<br />
cyclohexylamines from 2,6-diketones via an aldolization-dehydrationconjugate reductionreductive<br />
amination cascade that is catalyzed by a chiral Brønsted acid and accelerated by <strong>the</strong><br />
achiral amine substrate, which is ultimately incorporated into <strong>the</strong> product. 2,6-diketone was<br />
treated with 1.0 equiv <strong>of</strong> an achiral amine, 2.0 equiv <strong>of</strong> a Hantzsch ester, and 10 mol % <strong>of</strong> a<br />
chiral Brønsted acid resulted in <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> corresponding cyclohexylamines with<br />
mediocre to good yield (35-79%) and with good to high diastereoselectivity (82-96%). Alkyl,<br />
aryl and sterically congested aryl substituted 2,6-diketones were reductively aminated with<br />
high stereoselectivities. [62]<br />
NHR 2<br />
+ NHR 2 + NHR<br />
X - 2<br />
X -<br />
R 1<br />
X<br />
OR 1<br />
R 1<br />
i-Pr<br />
i-Pr<br />
Y<br />
i-Pr<br />
O<br />
O<br />
OOH<br />
P EtO 2 C CO 2 Et<br />
i-Pr<br />
N<br />
O<br />
H<br />
3(2.2equiv)<br />
i-Pr i-Pr<br />
R 2 NH 2 (1.5 equiv)<br />
(R)-TRIP (10mol%)<br />
MS 5Å, cyclohexane, 50<br />
OR o C<br />
1<br />
Y<br />
HN R 2<br />
R 1<br />
Scheme 3.21. Syn<strong>the</strong>sis <strong>of</strong> Pharmaceutical Building Blocks Utilizing Reductive Amination.<br />
Menche have also demonstrated that thiourea acts as an efficient organocatalyst <strong>for</strong> <strong>the</strong><br />
reductive amination <strong>of</strong> aldehydes using aniline derivative and <strong>the</strong> ethyl Hantzsch ester<br />
providing <strong>the</strong> corresponding achiral N-benzylanilines in good to excellent yields (72-93%).<br />
Using 1.1 equiv <strong>of</strong> Hantzsch ester and 1.0 equiv <strong>of</strong> thiourea with molecular sieves in toluene<br />
at 70 °C <strong>for</strong> 24 h substituted benzaldehydes as well as two aliphatic aldehydes were reacted<br />
with p-anisidine <strong>for</strong>ming <strong>the</strong> secondary amines in good isolated yields (scheme 3.22). [63]<br />
71