Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
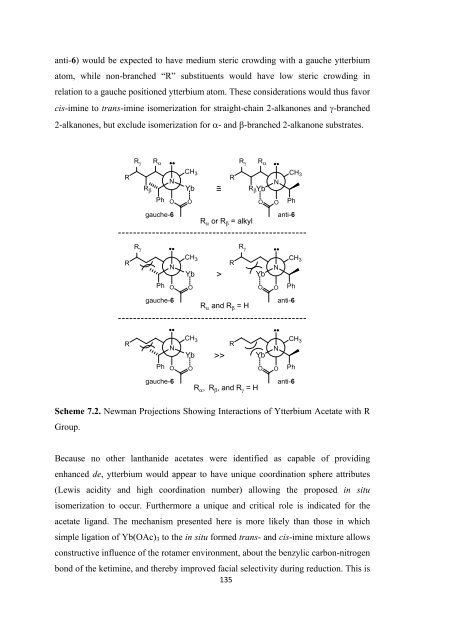
anti-6) would be expected to have medium steric crowding with a gauche ytterbium<br />
atom, while non-branched “R” substituents would have low steric crowding in<br />
relation to a gauche positioned ytterbium atom. These considerations would thus favor<br />
cis-imine to trans-imine isomerization <strong>for</strong> straight-chain 2-alkanones and γ-branched<br />
2-alkanones, but exclude isomerization <strong>for</strong> α- and β-branched 2-alkanone substrates.<br />
R γ<br />
R α<br />
R α<br />
R<br />
R β<br />
Ph<br />
N<br />
O<br />
CH 3<br />
Yb<br />
O<br />
~=<br />
R<br />
R γ<br />
R β<br />
Yb<br />
O<br />
N<br />
O<br />
CH 3<br />
Ph<br />
gauche-6<br />
R α or R β = alkyl<br />
anti-6<br />
R γ<br />
R γ<br />
R<br />
Ph<br />
N<br />
O<br />
CH 3<br />
Yb<br />
O<br />
><br />
R<br />
Yb<br />
O<br />
N<br />
O<br />
CH 3<br />
Ph<br />
gauche-6<br />
R α and R β = H<br />
anti-6<br />
R<br />
Ph<br />
N<br />
O<br />
CH 3<br />
Yb<br />
O<br />
>><br />
R<br />
Yb<br />
O<br />
N<br />
O<br />
CH 3<br />
Ph<br />
gauche-6<br />
R α , R β , and R γ = H<br />
anti-6<br />
Scheme 7.2. Newman Projections Showing Interactions <strong>of</strong> Ytterbium Acetate with R<br />
Group.<br />
Because no o<strong>the</strong>r lanthanide acetates were identified as capable <strong>of</strong> providing<br />
enhanced de, ytterbium would appear to have unique coordination sphere attributes<br />
(Lewis acidity and high coordination number) allowing <strong>the</strong> proposed in situ<br />
isomerization to occur. Fur<strong>the</strong>rmore a unique and critical role is indicated <strong>for</strong> <strong>the</strong><br />
acetate ligand. The mechanism presented here is more likely than those in which<br />
simple ligation <strong>of</strong> Yb(OAc) 3 to <strong>the</strong> in situ <strong>for</strong>med trans- and cis-imine mixture allows<br />
constructive influence <strong>of</strong> <strong>the</strong> rotamer environment, about <strong>the</strong> benzylic carbon-nitrogen<br />
bond <strong>of</strong> <strong>the</strong> ketimine, and <strong>the</strong>reby improved facial selectivity during reduction. This is<br />
135