Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
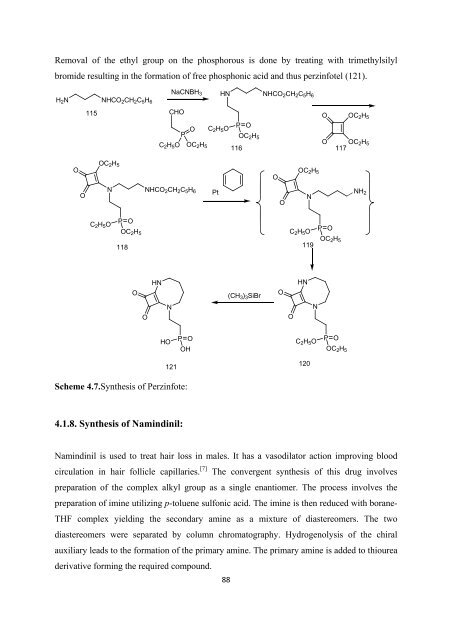
Removal <strong>of</strong> <strong>the</strong> ethyl group on <strong>the</strong> phosphorous is done by treating with trimethylsilyl<br />
bromide resulting in <strong>the</strong> <strong>for</strong>mation <strong>of</strong> free phosphonic acid and thus perzinfotel (121).<br />
NaCNBH 3 HN NHCO2 CH 2 C 5 H 6<br />
H 2 N NHCO 2 CH 2 C 5 H 6<br />
C 2 H 5 O<br />
115<br />
CHO<br />
O C<br />
P<br />
2 H 5 O<br />
O<br />
P<br />
OC 2 H 5<br />
OC 2 H 5<br />
116<br />
O<br />
O<br />
OC 2 H 5<br />
OC 2 H 5<br />
117<br />
O<br />
OC 2 H 5<br />
O<br />
OC 2 H 5<br />
O<br />
N NHCO 2 CH 2 C 5 H 6<br />
Pt<br />
O<br />
N<br />
NH 2<br />
C 2 H 5 O<br />
P O<br />
OC 2 H 5<br />
118<br />
C 2 H 5 O<br />
119<br />
P<br />
O<br />
OC 2 H 5<br />
O<br />
O<br />
HN<br />
N<br />
(CH 3 ) 3 SiBr<br />
O<br />
O<br />
HN<br />
N<br />
HO<br />
P<br />
O<br />
OH<br />
C 2 H 5 O<br />
P<br />
O<br />
OC 2 H 5<br />
121<br />
120<br />
Scheme 4.7.Syn<strong>the</strong>sis <strong>of</strong> Perzinfote:<br />
4.1.8. Syn<strong>the</strong>sis <strong>of</strong> Namindinil:<br />
Namindinil is used to treat hair loss in males. It has a vasodilator action improving blood<br />
circulation in hair follicle capillaries. [7] The convergent syn<strong>the</strong>sis <strong>of</strong> this drug involves<br />
preparation <strong>of</strong> <strong>the</strong> complex alkyl group as a single enantiomer. The process involves <strong>the</strong><br />
preparation <strong>of</strong> imine utilizing p-toluene sulfonic acid. The imine is <strong>the</strong>n reduced with borane-<br />
THF complex yielding <strong>the</strong> secondary amine as a mixture <strong>of</strong> diastereomers. The two<br />
diastereomers were separated by column chromatography. Hydrogenolysis <strong>of</strong> <strong>the</strong> chiral<br />
auxiliary leads to <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> primary amine. The primary amine is added to thiourea<br />
derivative <strong>for</strong>ming <strong>the</strong> required compound.<br />
88