Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
have to be faster than that <strong>of</strong> <strong>the</strong> o<strong>the</strong>r, resulting in an enantiomerically enriched product<br />
which is ano<strong>the</strong>r successful example <strong>of</strong> a dynamic kinetic resolution process. [60]<br />
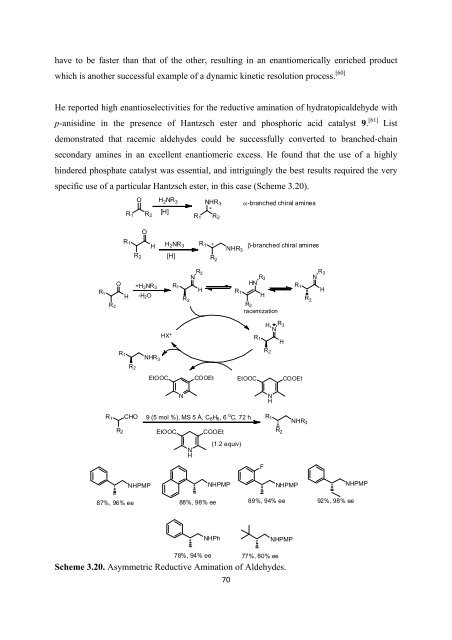
He reported high enantioselectivities <strong>for</strong> <strong>the</strong> reductive amination <strong>of</strong> hydratopicaldehyde with<br />
p-anisidine in <strong>the</strong> presence <strong>of</strong> Hantzsch ester and phosphoric acid catalyst 9. [61] List<br />
demonstrated that racemic aldehydes could be successfully converted to branched-chain<br />
secondary amines in an excellent enantiomeric excess. He found that <strong>the</strong> use <strong>of</strong> a highly<br />
hindered phosphate catalyst was essential, and intriguingly <strong>the</strong> best results required <strong>the</strong> very<br />
specific use <strong>of</strong> a particular Hantzsch ester, in this case (Scheme 3.20).<br />
O H 2 NR 3<br />
R 1 R 2<br />
[H]<br />
O<br />
R 1<br />
H H 2 NR 3<br />
R 2 [H]<br />
NHR 3<br />
R 1<br />
∗R 2<br />
α-branched chiral amines<br />
R 1 ∗<br />
β-branched chiral amines<br />
NHR 3<br />
R 2<br />
R 1<br />
R 2<br />
O<br />
H<br />
+H 2 NR 3<br />
-H 2 O<br />
R 1<br />
R 3<br />
N<br />
R 3<br />
HN<br />
H<br />
R 1<br />
H<br />
R 2<br />
R 2<br />
racemization<br />
R 3<br />
N<br />
R 1<br />
R 2<br />
H<br />
H<br />
N<br />
H<br />
HX*<br />
R 3<br />
N<br />
R 1<br />
R 2<br />
H<br />
R 1<br />
NHR 3<br />
R 2<br />
EtOOC<br />
COOEt<br />
EtOOC<br />
COOEt<br />
N<br />
R CHO 9(5mol%),MS5Å,C 6 H 6 ,6 o 1 C, 72 h R 1<br />
NHR 3<br />
R 2 R 2<br />
EtOOC<br />
COOEt<br />
N<br />
H<br />
(1.2 equiv)<br />
F<br />
NHPMP<br />
NHPMP<br />
NHPMP<br />
NHPMP<br />
87%, 96% ee<br />
88%, 98% ee<br />
89%, 94% ee<br />
92%, 98% ee<br />
NHPh<br />
NHPMP<br />
78%, 94% ee 77%, 80% ee<br />
Scheme 3.20. Asymmetric Reductive Amination <strong>of</strong> Aldehydes.<br />
70