Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
O<br />
NH 2<br />
1. p-TsOH/toluene<br />
2. BH 3 -THF/THF<br />
HN<br />
1.Column<br />
Chromatograpgy<br />
2.10 mol% Pd-C/EtOH<br />
NH 2<br />
N<br />
OH<br />
N<br />
N<br />
NH 2<br />
S<br />
C<br />
N<br />
NaCN<br />
NC<br />
S<br />
NH<br />
NH<br />
NC<br />
N<br />
H<br />
N<br />
NH<br />
NH 2<br />
CN<br />
CN<br />
CN<br />
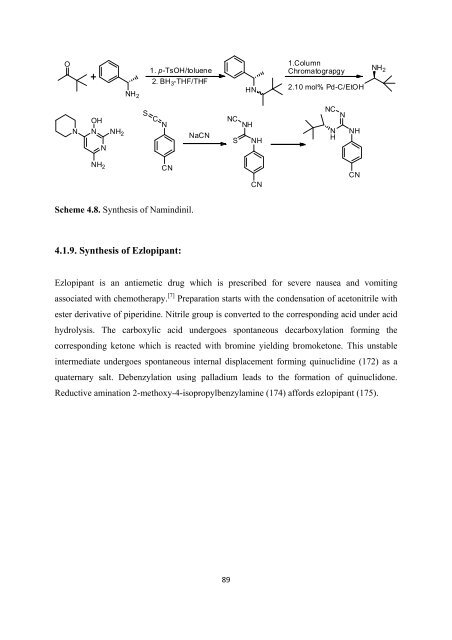
Scheme 4.8. Syn<strong>the</strong>sis <strong>of</strong> Namindinil.<br />
4.1.9. Syn<strong>the</strong>sis <strong>of</strong> Ezlopipant:<br />
Ezlopipant is an antiemetic drug which is prescribed <strong>for</strong> severe nausea and vomiting<br />
associated with chemo<strong>the</strong>rapy. [7] <strong>Preparation</strong> starts with <strong>the</strong> condensation <strong>of</strong> acetonitrile with<br />
ester derivative <strong>of</strong> piperidine. Nitrile group is converted to <strong>the</strong> corresponding acid under acid<br />
hydrolysis. The carboxylic acid undergoes spontaneous decarboxylation <strong>for</strong>ming <strong>the</strong><br />
corresponding ketone which is reacted with bromine yielding bromoketone. This unstable<br />
intermediate undergoes spontaneous internal displacement <strong>for</strong>ming quinuclidine (172) as a<br />
quaternary salt. Debenzylation using palladium leads to <strong>the</strong> <strong>for</strong>mation <strong>of</strong> quinuclidone.<br />
Reductive amination 2-methoxy-4-isopropylbenzylamine (174) af<strong>for</strong>ds ezlopipant (175).<br />
89