Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
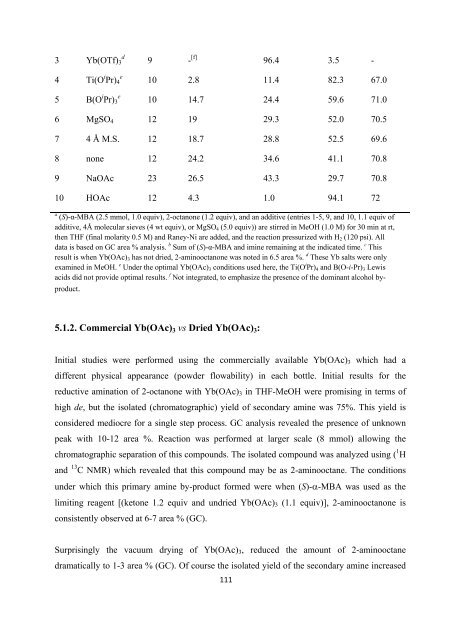
3 Yb(OTf) 3<br />
d<br />
4 Ti(O i Pr) 4<br />
e<br />
5 B(O i Pr) 3<br />
e<br />
9 - [f] 96.4 3.5 -<br />
10 2.8 11.4 82.3 67.0<br />
10 14.7 24.4 59.6 71.0<br />
6 MgSO 4 12 19 29.3 52.0 70.5<br />
7 4 Å M.S. 12 18.7 28.8 52.5 69.6<br />
8 none 12 24.2 34.6 41.1 70.8<br />
9 NaOAc 23 26.5 43.3 29.7 70.8<br />
10 HOAc 12 4.3 1.0 94.1 72<br />
a (S)-α-MBA (2.5 mmol, 1.0 equiv), 2-octanone (1.2 equiv), and an additive (entries 1-5, 9, and 10, 1.1 equiv <strong>of</strong><br />
additive, 4Å molecular sieves (4 wt equiv), or MgSO 4 (5.0 equiv)) are stirred in MeOH (1.0 M) <strong>for</strong> 30 min at rt,<br />
<strong>the</strong>n THF (final molarity 0.5 M) and Raney-Ni are added, and <strong>the</strong> reaction pressurized with H 2 (120 psi). All<br />
data is based on GC area % analysis. b Sum <strong>of</strong> (S)-α-MBA and imine remaining at <strong>the</strong> indicated time. c This<br />
result is when Yb(OAc) 3 has not dried, 2-aminooctanone was noted in 6.5 area %. d These Yb salts were only<br />
examined in MeOH. e Under <strong>the</strong> optimal Yb(OAc) 3 conditions used here, <strong>the</strong> Ti(O i Pr) 4 and B(O-i-Pr) 3 Lewis<br />
acids did not provide optimal results. f Not integrated, to emphasize <strong>the</strong> presence <strong>of</strong> <strong>the</strong> dominant alcohol byproduct.<br />
5.1.2. Commercial Yb(OAc) 3 vs Dried Yb(OAc) 3 :<br />
Initial studies were per<strong>for</strong>med using <strong>the</strong> commercially available Yb(OAc) 3 which had a<br />
different physical appearance (powder flowability) in each bottle. Initial results <strong>for</strong> <strong>the</strong><br />
reductive amination <strong>of</strong> 2-octanone with Yb(OAc) 3 in THF-MeOH were promising in terms <strong>of</strong><br />
high de, but <strong>the</strong> isolated (chromatographic) yield <strong>of</strong> secondary amine was 75%. This yield is<br />
considered mediocre <strong>for</strong> a single step process. GC analysis revealed <strong>the</strong> presence <strong>of</strong> unknown<br />
peak with 10-12 area %. Reaction was per<strong>for</strong>med at larger scale (8 mmol) allowing <strong>the</strong><br />
chromatographic separation <strong>of</strong> this compounds. The isolated compound was analyzed using ( 1 H<br />
and 13 C NMR) which revealed that this compound may be as 2-aminooctane. The conditions<br />
under which this primary amine by-product <strong>for</strong>med were when (S)-α-MBA was used as <strong>the</strong><br />
limiting reagent [(ketone 1.2 equiv and undried Yb(OAc) 3 (1.1 equiv)], 2-aminooctanone is<br />
consistently observed at 6-7 area % (GC).<br />
Surprisingly <strong>the</strong> vacuum drying <strong>of</strong> Yb(OAc) 3 , reduced <strong>the</strong> amount <strong>of</strong> 2-aminooctane<br />
dramatically to 1-3 area % (GC). Of course <strong>the</strong> isolated yield <strong>of</strong> <strong>the</strong> secondary amine increased<br />
111