Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
CO 2 C 2 H 5<br />
N<br />
CN<br />
base<br />
CN<br />
O H + N<br />
O<br />
N<br />
167 168<br />
169<br />
170<br />
Br 2<br />
N<br />
O<br />
H 2<br />
N<br />
O<br />
O<br />
Br<br />
173<br />
N<br />
172<br />
OCH 3<br />
171<br />
174<br />
NH 2<br />
N<br />
NH<br />
OCH 3<br />
175<br />
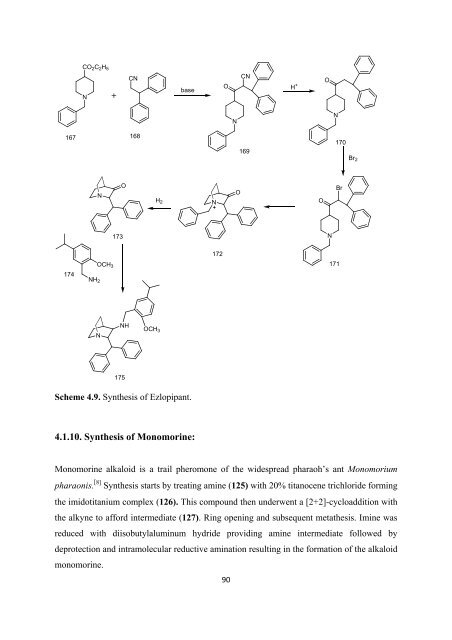
Scheme 4.9. Syn<strong>the</strong>sis <strong>of</strong> Ezlopipant.<br />
4.1.10. Syn<strong>the</strong>sis <strong>of</strong> Monomorine:<br />
Monomorine alkaloid is a trail pheromone <strong>of</strong> <strong>the</strong> widespread pharaoh’s ant Monomorium<br />
pharaonis. [8] Syn<strong>the</strong>sis starts by treating amine (125) with 20% titanocene trichloride <strong>for</strong>ming<br />
<strong>the</strong> imidotitanium complex (126). This compound <strong>the</strong>n underwent a [2+2]-cycloaddition with<br />
<strong>the</strong> alkyne to af<strong>for</strong>d intermediate (127). Ring opening and subsequent meta<strong>the</strong>sis. Imine was<br />
reduced with diisobutylaluminum hydride providing amine intermediate followed by<br />
deprotection and intramolecular reductive amination resulting in <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong> alkaloid<br />
monomorine.<br />
90