Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
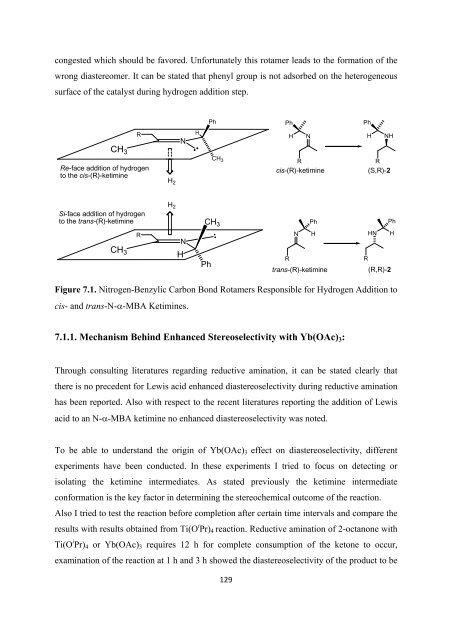
congested which should be favored. Un<strong>for</strong>tunately this rotamer leads to <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong><br />
wrong diastereomer. It can be stated that phenyl group is not adsorbed on <strong>the</strong> heterogeneous<br />
surface <strong>of</strong> <strong>the</strong> catalyst during hydrogen addition step.<br />
Ph<br />
Ph<br />
Ph<br />
R H<br />
N<br />
CH 3<br />
H<br />
N<br />
H<br />
NH<br />
Re-face addition <strong>of</strong> hydrogen<br />
to <strong>the</strong> cis-(R)-ketimine<br />
H 2<br />
CH 3<br />
R<br />
cis-(R)-ketimine<br />
R<br />
(S,R)-2<br />
Si-face addition <strong>of</strong> hydrogen<br />
to <strong>the</strong> trans-(R)-ketimine<br />
H 2<br />
CH 3<br />
Ph<br />
Ph<br />
CH 3<br />
R<br />
N<br />
H<br />
Ph<br />
N H<br />
R<br />
trans-(R)-ketimine<br />
HN H<br />
R<br />
(R,R)-2<br />
Figure 7.1. Nitrogen-Benzylic Carbon Bond Rotamers Responsible <strong>for</strong> Hydrogen Addition to<br />
cis- and trans-N-α-MBA Ketimines.<br />
7.1.1. Mechanism Behind Enhanced Stereoselectivity with Yb(OAc) 3 :<br />
Through consulting literatures regarding reductive amination, it can be stated clearly that<br />
<strong>the</strong>re is no precedent <strong>for</strong> Lewis acid enhanced diastereoselectivity during reductive amination<br />
has been reported. Also with respect to <strong>the</strong> recent literatures reporting <strong>the</strong> addition <strong>of</strong> Lewis<br />
acid to an N-α-MBA ketimine no enhanced diastereoselectivity was noted.<br />
To be able to understand <strong>the</strong> origin <strong>of</strong> Yb(OAc) 3 effect on diastereoselectivity, different<br />
experiments have been conducted. In <strong>the</strong>se experiments I tried to focus on detecting or<br />
isolating <strong>the</strong> ketimine intermediates. As stated previously <strong>the</strong> ketimine intermediate<br />
con<strong>for</strong>mation is <strong>the</strong> key factor in determining <strong>the</strong> stereochemical outcome <strong>of</strong> <strong>the</strong> reaction.<br />
Also I tried to test <strong>the</strong> reaction be<strong>for</strong>e completion after certain time intervals and compare <strong>the</strong><br />
results with results obtained from Ti(O i Pr) 4 reaction. Reductive amination <strong>of</strong> 2-octanone with<br />
Ti(O i Pr) 4 or Yb(OAc) 3 requires 12 h <strong>for</strong> complete consumption <strong>of</strong> <strong>the</strong> ketone to occur,<br />
examination <strong>of</strong> <strong>the</strong> reaction at 1 h and 3 h showed <strong>the</strong> diastereoselectivity <strong>of</strong> <strong>the</strong> product to be<br />
129