Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
catalysts <strong>for</strong> reductive amination in 1974. He tested rhodium and cobalt based catalysts <strong>for</strong><br />
<strong>the</strong> reductive alkylation <strong>of</strong> ammonia and aniline derivatives. He found that <strong>the</strong> activity <strong>of</strong><br />
cobalt catalyst is highly influenced by <strong>the</strong> structure <strong>of</strong> phosphine ligand. Also he recognized<br />
that using basic aliphatic amines led to poising <strong>of</strong> <strong>the</strong> cobalt catalyst and no product was<br />
<strong>for</strong>med. On <strong>the</strong> o<strong>the</strong>r hand rhodium was used successfully in <strong>the</strong> reductive amination <strong>of</strong> <strong>the</strong>se<br />
basic amines. [13] Despite <strong>the</strong>se interesting results <strong>the</strong> reaction conditions were harsh (100–300<br />
bar H 2 , 110–200 °C) and no turnover number were reported. In 2000, Börner described more<br />
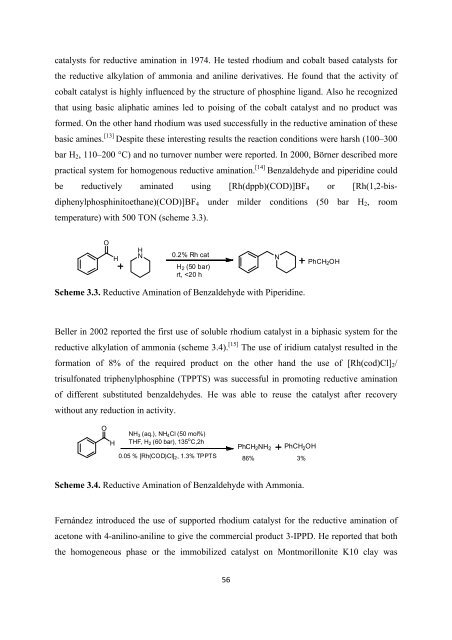
practical system <strong>for</strong> homogenous reductive amination. [14] Benzaldehyde and piperidine could<br />
be reductively aminated using [Rh(dppb)(COD)]BF 4 or [Rh(1,2-bisdiphenylphosphinitoethane)(COD)]BF<br />
4 under milder conditions (50 bar H 2 , room<br />
temperature) with 500 TON (scheme 3.3).<br />
O<br />
H<br />
H<br />
N<br />
0.2% Rh cat<br />
H 2 (50 bar)<br />
rt,