Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
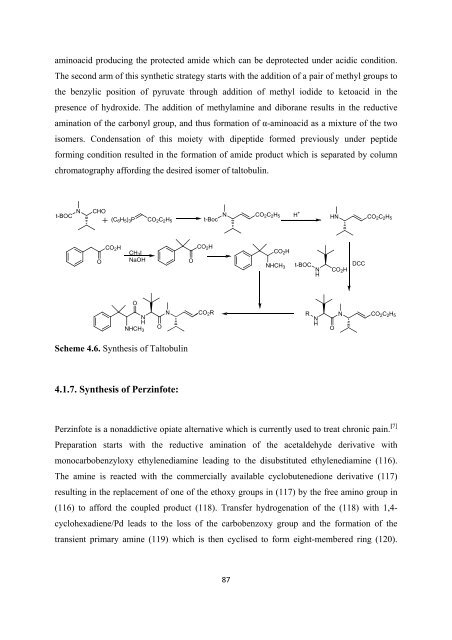
aminoacid producing <strong>the</strong> protected amide which can be deprotected under acidic condition.<br />
The second arm <strong>of</strong> this syn<strong>the</strong>tic strategy starts with <strong>the</strong> addition <strong>of</strong> a pair <strong>of</strong> methyl groups to<br />
<strong>the</strong> benzylic position <strong>of</strong> pyruvate through addition <strong>of</strong> methyl iodide to ketoacid in <strong>the</strong><br />
presence <strong>of</strong> hydroxide. The addition <strong>of</strong> methylamine and diborane results in <strong>the</strong> reductive<br />
amination <strong>of</strong> <strong>the</strong> carbonyl group, and thus <strong>for</strong>mation <strong>of</strong> α-aminoacid as a mixture <strong>of</strong> <strong>the</strong> two<br />
isomers. Condensation <strong>of</strong> this moiety with dipeptide <strong>for</strong>med previously under peptide<br />
<strong>for</strong>ming condition resulted in <strong>the</strong> <strong>for</strong>mation <strong>of</strong> amide product which is separated by column<br />
chromatography af<strong>for</strong>ding <strong>the</strong> desired isomer <strong>of</strong> taltobulin.<br />
t-BOC<br />
N<br />
CHO<br />
N<br />
(C 6 H 5 ) 3 P CO 2 C 2 H 5 t-Boc<br />
CO 2 C 2 H 5 H +<br />
HN CO2 C 2 H 5<br />
O<br />
CO 2 H<br />
CH 3 I<br />
NaOH<br />
O<br />
CO 2 H<br />
CO 2 H<br />
NHCH 3<br />
t-BOC<br />
N<br />
H<br />
CO 2 H<br />
DCC<br />
O<br />
N<br />
H<br />
NHCH 3<br />
O<br />
N<br />
CO 2 R<br />
R<br />
N<br />
H<br />
O<br />
N CO 2 C 2 H 5<br />
Scheme 4.6. Syn<strong>the</strong>sis <strong>of</strong> Taltobulin<br />
4.1.7. Syn<strong>the</strong>sis <strong>of</strong> Perzinfote:<br />
Perzinfote is a nonaddictive opiate alternative which is currently used to treat chronic pain. [7]<br />
<strong>Preparation</strong> starts with <strong>the</strong> reductive amination <strong>of</strong> <strong>the</strong> acetaldehyde derivative with<br />
monocarbobenzyloxy ethylenediamine leading to <strong>the</strong> disubstituted ethylenediamine (116).<br />
The amine is reacted with <strong>the</strong> commercially available cyclobutenedione derivative (117)<br />
resulting in <strong>the</strong> replacement <strong>of</strong> one <strong>of</strong> <strong>the</strong> ethoxy groups in (117) by <strong>the</strong> free amino group in<br />
(116) to af<strong>for</strong>d <strong>the</strong> coupled product (118). Transfer hydrogenation <strong>of</strong> <strong>the</strong> (118) with 1,4-<br />
cyclohexadiene/Pd leads to <strong>the</strong> loss <strong>of</strong> <strong>the</strong> carbobenzoxy group and <strong>the</strong> <strong>for</strong>mation <strong>of</strong> <strong>the</strong><br />
transient primary amine (119) which is <strong>the</strong>n cyclised to <strong>for</strong>m eight-membered ring (120).<br />
87