Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
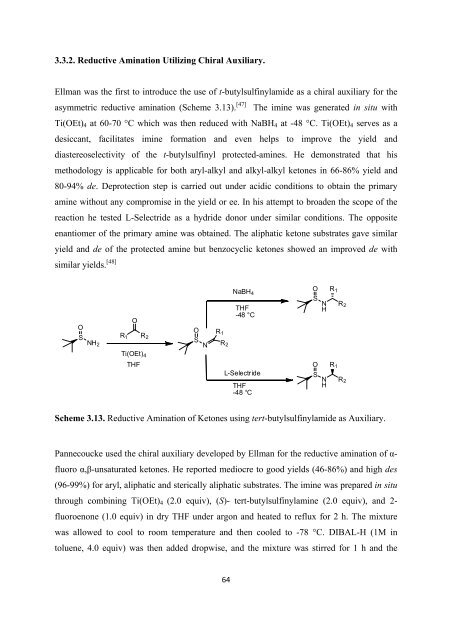
3.3.2. Reductive Amination Utilizing <strong>Chiral</strong> Auxiliary.<br />
Ellman was <strong>the</strong> first to introduce <strong>the</strong> use <strong>of</strong> t-butylsulfinylamide as a chiral auxiliary <strong>for</strong> <strong>the</strong><br />
asymmetric reductive amination (Scheme 3.13). [47] The imine was generated in situ with<br />
Ti(OEt) 4 at 60-70 °C which was <strong>the</strong>n reduced with NaBH 4 at -48 °C. Ti(OEt) 4 serves as a<br />
desiccant, facilitates imine <strong>for</strong>mation and even helps to improve <strong>the</strong> yield and<br />
diastereoselectivity <strong>of</strong> <strong>the</strong> t-butylsulfinyl protected-amines. He demonstrated that his<br />
methodology is applicable <strong>for</strong> both aryl-alkyl and alkyl-alkyl ketones in 66-86% yield and<br />
80-94% de. Deprotection step is carried out under acidic conditions to obtain <strong>the</strong> primary<br />
amine without any compromise in <strong>the</strong> yield or ee. In his attempt to broaden <strong>the</strong> scope <strong>of</strong> <strong>the</strong><br />
reaction he tested L-Selectride as a hydride donor under similar conditions. The opposite<br />
enantiomer <strong>of</strong> <strong>the</strong> primary amine was obtained. The aliphatic ketone substrates gave similar<br />
yield and de <strong>of</strong> <strong>the</strong> protected amine but benzocyclic ketones showed an improved de with<br />
similar yields. [48]<br />
O<br />
S NH2<br />
O<br />
R 1 R 2<br />
NaBH 4<br />
THF<br />
-48 °C<br />
O R 1<br />
S N R 2<br />
O R 1<br />
S NH R 2<br />
Ti(OEt) 4<br />
THF<br />
L-Selectride<br />
O<br />
R 1<br />
S NH R 2<br />
THF<br />
-48 °C<br />
Scheme 3.13. Reductive Amination <strong>of</strong> Ketones using tert-butylsulfinylamide as Auxiliary.<br />
Pannecoucke used <strong>the</strong> chiral auxiliary developed by Ellman <strong>for</strong> <strong>the</strong> reductive amination <strong>of</strong> α-<br />
fluoro α,β-unsaturated ketones. He reported mediocre to good yields (46-86%) and high des<br />
(96-99%) <strong>for</strong> aryl, aliphatic and sterically aliphatic substrates. The imine was prepared in situ<br />
through combining Ti(OEt) 4 (2.0 equiv), (S)- tert-butylsulfinylamine (2.0 equiv), and 2-<br />
fluoroenone (1.0 equiv) in dry THF under argon and heated to reflux <strong>for</strong> 2 h. The mixture<br />
was allowed to cool to room temperature and <strong>the</strong>n cooled to -78 °C. DIBAL-H (1M in<br />
toluene, 4.0 equiv) was <strong>the</strong>n added dropwise, and <strong>the</strong> mixture was stirred <strong>for</strong> 1 h and <strong>the</strong><br />
64