Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
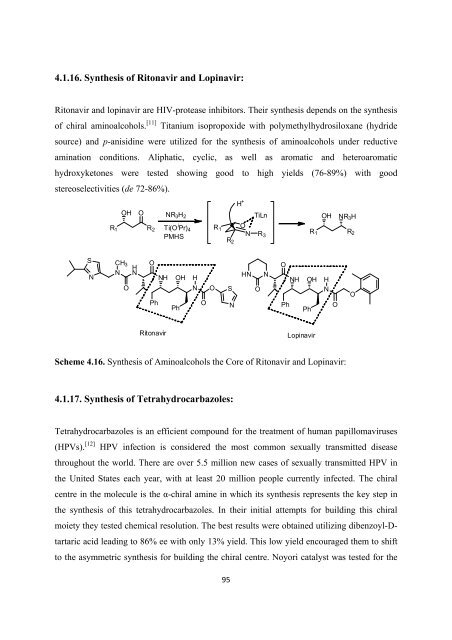
4.1.16. Syn<strong>the</strong>sis <strong>of</strong> Ritonavir and Lopinavir:<br />
Ritonavir and lopinavir are HIV-protease inhibitors. Their syn<strong>the</strong>sis depends on <strong>the</strong> syn<strong>the</strong>sis<br />
<strong>of</strong> chiral aminoalcohols. [11] Titanium isopropoxide with polymethylhydrosiloxane (hydride<br />
source) and p-anisidine were utilized <strong>for</strong> <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> aminoalcohols under reductive<br />
amination conditions. Aliphatic, cyclic, as well as aromatic and heteroaromatic<br />
hydroxyketones were tested showing good to high yields (76-89%) with good<br />
stereoselectivities (de 72-86%).<br />
R 1<br />
OH O<br />
R 2<br />
NR 3 H 2<br />
Ti(O i Pr) 4<br />
PMHS<br />
R 1<br />
O<br />
N<br />
R 2<br />
H + TiLn<br />
OH NR 3 H<br />
R 1<br />
R 3<br />
R 2<br />
S<br />
N<br />
CH 3<br />
N<br />
O<br />
H<br />
N<br />
O<br />
Ph<br />
NH<br />
OH<br />
Ph<br />
H<br />
N<br />
O<br />
O<br />
S<br />
N<br />
HN<br />
O<br />
N<br />
O<br />
Ph<br />
NH<br />
OH<br />
Ph<br />
H<br />
N<br />
O<br />
O<br />
Ritonavir<br />
Lopinavir<br />
Scheme 4.16. Syn<strong>the</strong>sis <strong>of</strong> Aminoalcohols <strong>the</strong> Core <strong>of</strong> Ritonavir and Lopinavir:<br />
4.1.17. Syn<strong>the</strong>sis <strong>of</strong> Tetrahydrocarbazoles:<br />
Tetrahydrocarbazoles is an efficient compound <strong>for</strong> <strong>the</strong> treatment <strong>of</strong> human papillomaviruses<br />
(HPVs). [12] HPV infection is considered <strong>the</strong> most common sexually transmitted disease<br />
throughout <strong>the</strong> world. There are over 5.5 million new cases <strong>of</strong> sexually transmitted HPV in<br />
<strong>the</strong> United States each year, with at least 20 million people currently infected. The chiral<br />
centre in <strong>the</strong> molecule is <strong>the</strong> α-chiral amine in which its syn<strong>the</strong>sis represents <strong>the</strong> key step in<br />
<strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> this tetrahydrocarbazoles. In <strong>the</strong>ir initial attempts <strong>for</strong> building this chiral<br />
moiety <strong>the</strong>y tested chemical resolution. The best results were obtained utilizing dibenzoyl-Dtartaric<br />
acid leading to 86% ee with only 13% yield. This low yield encouraged <strong>the</strong>m to shift<br />
to <strong>the</strong> asymmetric syn<strong>the</strong>sis <strong>for</strong> building <strong>the</strong> chiral centre. Noyori catalyst was tested <strong>for</strong> <strong>the</strong><br />
95