Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
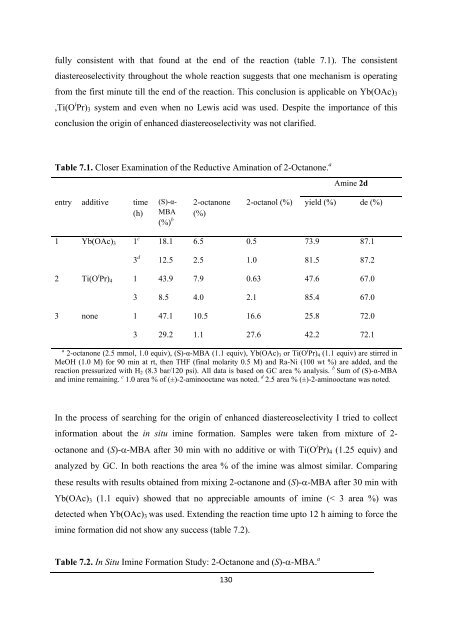
Table 7.1. Closer Examination <strong>of</strong> <strong>the</strong> Reductive Amination <strong>of</strong> 2-Octanone. a Amine 2d<br />
fully consistent with that found at <strong>the</strong> end <strong>of</strong> <strong>the</strong> reaction (table 7.1). The consistent<br />
diastereoselectivity throughout <strong>the</strong> whole reaction suggests that one mechanism is operating<br />
from <strong>the</strong> first minute till <strong>the</strong> end <strong>of</strong> <strong>the</strong> reaction. This conclusion is applicable on Yb(OAc) 3<br />
,Ti(O i Pr) 3 system and even when no Lewis acid was used. Despite <strong>the</strong> importance <strong>of</strong> this<br />
conclusion <strong>the</strong> origin <strong>of</strong> enhanced diastereoselectivity was not clarified.<br />
entry additive time<br />
(h)<br />
2-octanone<br />
(S)-α-<br />
(%) b<br />
MBA (%)<br />
2-octanol (%) yield (%) de (%)<br />
1 Yb(OAc) 3 1 c<br />
3 d 18.1<br />
12.5<br />
6.5<br />
2.5<br />
0.5<br />
1.0<br />
73.9<br />
81.5<br />
87.1<br />
87.2<br />
2 Ti(O i Pr) 4 1<br />
43.9<br />
7.9<br />
0.63<br />
47.6<br />
67.0<br />
3<br />
8.5<br />
4.0<br />
2.1<br />
85.4<br />
67.0<br />
3 none 1<br />
47.1<br />
10.5<br />
16.6<br />
25.8<br />
72.0<br />
3<br />
29.2<br />
1.1<br />
27.6<br />
42.2<br />
72.1<br />
a 2-octanone (2.5 mmol, 1.0 equiv), (S)-α-MBA (1.1 equiv), Yb(OAc) 3 or Ti(O i Pr) 4 (1.1 equiv) are stirred in<br />
MeOH (1.0 M) <strong>for</strong> 90 min at rt, <strong>the</strong>n THF (final molarity 0.5 M) and Ra-Ni (100 wt %) are added, and <strong>the</strong><br />
reaction pressurized with H 2 (8.3 bar/120 psi). All data is based on GC area % analysis. b Sum <strong>of</strong> (S)-α-MBA<br />
and imine remaining. c 1.0 area % <strong>of</strong> (±)-2-aminooctane was noted. d 2.5 area % (±)-2-aminooctane was noted.<br />
In <strong>the</strong> process <strong>of</strong> searching <strong>for</strong> <strong>the</strong> origin <strong>of</strong> enhanced diastereoselectivity I tried to collect<br />
in<strong>for</strong>mation about <strong>the</strong> in situ imine <strong>for</strong>mation. Samples were taken from mixture <strong>of</strong> 2-<br />
octanone and (S)-α-MBA after 30 min with no additive or with Ti(O i Pr) 4 (1.25 equiv) and<br />
analyzed by GC. In both reactions <strong>the</strong> area % <strong>of</strong> <strong>the</strong> imine was almost similar. Comparing<br />
<strong>the</strong>se results with results obtained from mixing 2-octanone and (S)-α-MBA after 30 min with<br />
Yb(OAc) 3 (1.1 equiv) showed that no appreciable amounts <strong>of</strong> imine (< 3 area %) was<br />
detected when Yb(OAc) 3 was used. Extending <strong>the</strong> reaction time upto 12 h aiming to <strong>for</strong>ce <strong>the</strong><br />
imine <strong>for</strong>mation did not show any success (table 7.2).<br />
Table 7.2. In Situ Imine Formation Study: 2-Octanone and (S)-α-MBA. a<br />
130