Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
2.2.3. Nguyen Special Substrates.<br />
Apart from <strong>the</strong> classical substrates (structure 1-3, figure 2.1) investigated, substrates which<br />
were tested by Nguyen were unique. By today’s standards <strong>the</strong> use <strong>of</strong> stoichiometric quantities<br />
<strong>of</strong> a chiral reducing agent are not acceptable, but in this case Nguyen has developed a system<br />
capable <strong>of</strong> accepting a much broader substrate scope and <strong>the</strong>rein lies <strong>the</strong> significance <strong>of</strong> his<br />
research. Using stoichiometric amounts <strong>of</strong> (S)-BINOL/AlMe 3 with isopropanol as a source <strong>of</strong><br />
hydrogen to reduce different N-phosphinoyl imines. Subtle difference between small alkyl<br />
groups could be distinguished. They reported 93% ee with 85% yield with imine derived<br />
from 3-octanone. This class <strong>of</strong> substrates is syn<strong>the</strong>sized utilizing carbanion chemistry because<br />
hydrogen reduction gives low ee (15%). As far as we know this is <strong>the</strong> only example <strong>for</strong><br />
reduction <strong>of</strong> 3-octanonene utilizing hydrogen with such high enantioselectivity. He tested his<br />
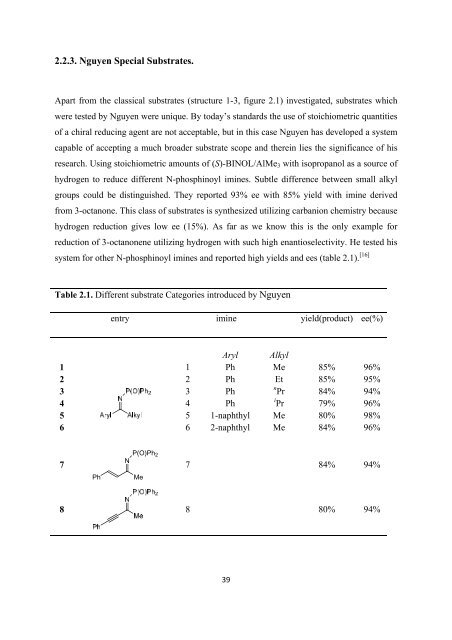
system <strong>for</strong> o<strong>the</strong>r N-phosphinoyl imines and reported high yields and ees (table 2.1). [16]<br />
Table 2.1. Different substrate Categories introduced by Nguyen<br />
entry imine yield(product) ee(%)<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
1<br />
2<br />
3<br />
4<br />
5<br />
6<br />
Aryl<br />
Ph<br />
Ph<br />
Ph<br />
Ph<br />
1-naphthyl<br />
2-naphthyl<br />
Alkyl<br />
Me<br />
Et<br />
n Pr<br />
i Pr<br />
Me<br />
Me<br />
85%<br />
85%<br />
84%<br />
79%<br />
80%<br />
84%<br />
96%<br />
95%<br />
94%<br />
96%<br />
98%<br />
96%<br />
7<br />
Ph<br />
N<br />
P(O)Ph 2<br />
7 84% 94%<br />
Me<br />
8 8 80% 94%<br />
39