Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
library <strong>of</strong> organocatalysts which were used efficiently <strong>for</strong> different organic trans<strong>for</strong>mations.<br />
Several research groups focused <strong>the</strong>ir ef<strong>for</strong>ts on <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> novel organocatalysts <strong>for</strong><br />
asymmetric imine reduction and reductive amination. Among <strong>the</strong>se trans<strong>for</strong>mations are imine<br />
reduction and reductive amination. At this time, development in <strong>the</strong> application <strong>of</strong><br />
organocatalysis <strong>for</strong> reductive amination is still in its infantile stage compared to o<strong>the</strong>r<br />
trans<strong>for</strong>mations. [56]<br />
X +2 [H]<br />
H<br />
X<br />
∗<br />
chiral<br />
catalyst R 1<br />
X=CR 2 ,O,NR<br />
R 1 R 2<br />
R 2<br />
Scheme 3.17. Asymmetric Reduction <strong>of</strong> prochiral Compounds.<br />
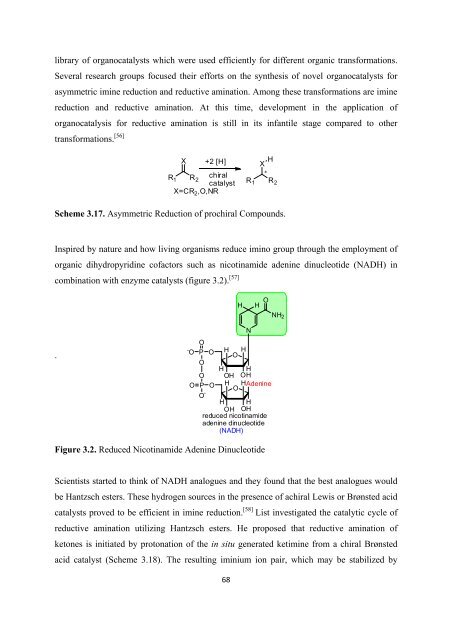
Inspired by nature and how living organisms reduce imino group through <strong>the</strong> employment <strong>of</strong><br />
organic dihydropyridine c<strong>of</strong>actors such as nicotinamide adenine dinucleotide (NADH) in<br />
combination with enzyme catalysts (figure 3.2). [57]<br />
H<br />
H<br />
O<br />
NH 2<br />
.<br />
- O<br />
O<br />
O<br />
P<br />
O<br />
O<br />
P<br />
O -<br />
O<br />
N<br />
H H<br />
O<br />
H H<br />
OH OH<br />
O H HAdenine<br />
O<br />
H H<br />
OH OH<br />
reduced nicotinamide<br />
adenine dinucleotide<br />
(NADH)<br />
Figure 3.2. Reduced Nicotinamide Adenine Dinucleotide<br />
Scientists started to think <strong>of</strong> NADH analogues and <strong>the</strong>y found that <strong>the</strong> best analogues would<br />
be Hantzsch esters. These hydrogen sources in <strong>the</strong> presence <strong>of</strong> achiral Lewis or Brønsted acid<br />
catalysts proved to be efficient in imine reduction. [58] List investigated <strong>the</strong> catalytic cycle <strong>of</strong><br />
reductive amination utilizing Hantzsch esters. He proposed that reductive amination <strong>of</strong><br />
ketones is initiated by protonation <strong>of</strong> <strong>the</strong> in situ generated ketimine from a chiral Brønsted<br />
acid catalyst (Scheme 3.18). The resulting iminium ion pair, which may be stabilized by<br />
68