Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
.<br />
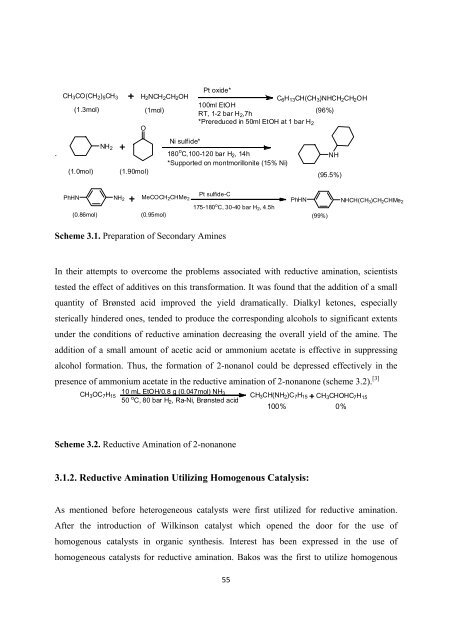
CH 3 CO(CH 2 ) 5 CH 3 H 2 NCH 2 CH 2 OH<br />
Pt oxide*<br />
C 6 H 13 CH(CH 3 )NHCH 2 CH 2 OH<br />
(1.3mol) (1mol)<br />
100ml EtOH<br />
RT, 1-2 bar H 2 ,7h<br />
(96%)<br />
*Prereduced in 50ml EtOH at 1 bar H 2<br />
O<br />
(1.0mol)<br />
NH 2<br />
(1.90mol)<br />
Ni sulfide*<br />
180 o C,100-120 bar H 2 ,14h<br />
*Supported on montmorillonite (15% Ni)<br />
NH<br />
(95.5%)<br />
PhHN NH 2 MeCOCH 2 CHMe 2<br />
Pt sulfide-C<br />
PhHN NHCH(CH 3 )CH 2 CHMe 2<br />
175-180 o C, 30-40 bar H 2 ,4.5h<br />
(0.86mol) (0.95mol)<br />
(99%)<br />
Scheme 3.1. <strong>Preparation</strong> <strong>of</strong> Secondary <strong>Amines</strong><br />
In <strong>the</strong>ir attempts to overcome <strong>the</strong> problems associated with reductive amination, scientists<br />
tested <strong>the</strong> effect <strong>of</strong> additives on this trans<strong>for</strong>mation. It was found that <strong>the</strong> addition <strong>of</strong> a small<br />
quantity <strong>of</strong> Brønsted acid improved <strong>the</strong> yield dramatically. Dialkyl ketones, especially<br />
sterically hindered ones, tended to produce <strong>the</strong> corresponding alcohols to significant extents<br />
under <strong>the</strong> conditions <strong>of</strong> reductive amination decreasing <strong>the</strong> overall yield <strong>of</strong> <strong>the</strong> amine. The<br />
addition <strong>of</strong> a small amount <strong>of</strong> acetic acid or ammonium acetate is effective in suppressing<br />
alcohol <strong>for</strong>mation. Thus, <strong>the</strong> <strong>for</strong>mation <strong>of</strong> 2-nonanol could be depressed effectively in <strong>the</strong><br />
presence <strong>of</strong> ammonium acetate in <strong>the</strong> reductive amination <strong>of</strong> 2-nonanone (scheme 3.2). [3]<br />
CH 3 OC 7 H 15<br />
10 mL EtOH/0.8 g (0.047mol) NH 3<br />
50 o C, 80 bar H 2 ,Ra-Ni,Brønstedacid<br />
CH 3 CH(NH 2 )C 7 H 15 CH 3 CHOHC 7 H 15<br />
100% 0%<br />
Scheme 3.2. Reductive Amination <strong>of</strong> 2-nonanone<br />
3.1.2. Reductive Amination Utilizing Homogenous Catalysis:<br />
As mentioned be<strong>for</strong>e heterogeneous catalysts were first utilized <strong>for</strong> reductive amination.<br />
After <strong>the</strong> introduction <strong>of</strong> Wilkinson catalyst which opened <strong>the</strong> door <strong>for</strong> <strong>the</strong> use <strong>of</strong><br />
homogenous catalysts in organic syn<strong>the</strong>sis. Interest has been expressed in <strong>the</strong> use <strong>of</strong><br />
homogeneous catalysts <strong>for</strong> reductive amination. Bakos was <strong>the</strong> first to utilize homogenous<br />
55