Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
Scheme 3.9. Syn<strong>the</strong>sis <strong>of</strong> (S)-Metolachlor<br />
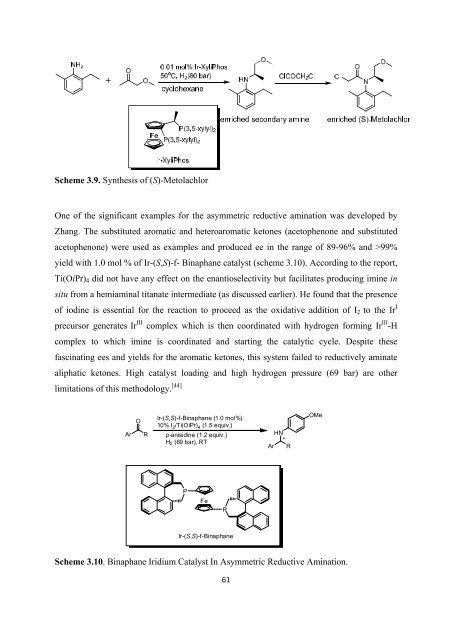
One <strong>of</strong> <strong>the</strong> significant examples <strong>for</strong> <strong>the</strong> asymmetric reductive amination was developed by<br />
Zhang. The substituted aromatic and heteroaromatic ketones (acetophenone and substituted<br />
acetophenone) were used as examples and produced ee in <strong>the</strong> range <strong>of</strong> 89-96% and >99%<br />
yield with 1.0 mol % <strong>of</strong> Ir-(S,S)-f- Binaphane catalyst (scheme 3.10). According to <strong>the</strong> report,<br />
Ti(OiPr) 4 did not have any effect on <strong>the</strong> enantioselectivity but facilitates producing imine in<br />
situ from a hemiaminal titanate intermediate (as discussed earlier). He found that <strong>the</strong> presence<br />
<strong>of</strong> iodine is essential <strong>for</strong> <strong>the</strong> reaction to proceed as <strong>the</strong> oxidative addition <strong>of</strong> I 2 to <strong>the</strong> Ir I<br />
precursor generates Ir III complex which is <strong>the</strong>n coordinated with hydrogen <strong>for</strong>ming Ir III -H<br />
complex to which imine is coordinated and starting <strong>the</strong> catalytic cycle. Despite <strong>the</strong>se<br />
fascinating ees and yields <strong>for</strong> <strong>the</strong> aromatic ketones, this system failed to reductively aminate<br />
aliphatic ketones. High catalyst loading and high hydrogen pressure (69 bar) are o<strong>the</strong>r<br />
limitations <strong>of</strong> this methodology. [44]<br />
Ar<br />
O<br />
R<br />
Ir-(S,S)-f-Binaphane (1.0 mol%)<br />
10% I 2 /Ti(OiPr) 4 (1.5 equiv.)<br />
p-anisidine (1.2 equiv.)<br />
H 2 (69 bar), RT<br />
HN<br />
∗<br />
Ar R<br />
OMe<br />
P<br />
Fe<br />
P<br />
Ir-(S,S)-f-Binaphane<br />
Scheme 3.10. Binaphane Iridium Catalyst In Asymmetric Reductive Amination.<br />
61