Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
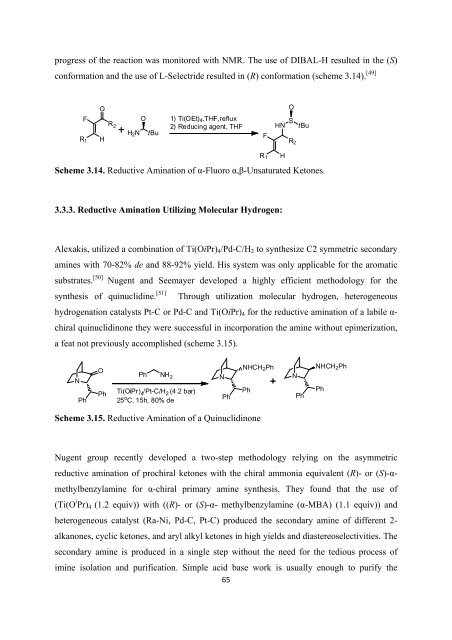
progress <strong>of</strong> <strong>the</strong> reaction was monitored with NMR. The use <strong>of</strong> DIBAL-H resulted in <strong>the</strong> (S)<br />
con<strong>for</strong>mation and <strong>the</strong> use <strong>of</strong> L-Selectride resulted in (R) con<strong>for</strong>mation (scheme 3.14). [49]<br />
F<br />
R 1<br />
O<br />
H<br />
R 2<br />
H 2 N<br />
O<br />
tBu<br />
1) Ti(OEt) 4 ,THF,reflux<br />
2) Reducing agent, THF<br />
F<br />
HN S O<br />
R 2<br />
tBu<br />
Scheme 3.14. Reductive Amination <strong>of</strong> α-Fluoro α,β-Unsaturated Ketones.<br />
R 1<br />
H<br />
3.3.3. Reductive Amination Utilizing Molecular Hydrogen:<br />
Alexakis, utilized a combination <strong>of</strong> Ti(OiPr) 4 /Pd-C/H 2 to syn<strong>the</strong>size C2 symmetric secondary<br />
amines with 70-82% de and 88-92% yield. His system was only applicable <strong>for</strong> <strong>the</strong> aromatic<br />
substrates. [50] Nugent and Seemayer developed a highly efficient methodology <strong>for</strong> <strong>the</strong><br />
syn<strong>the</strong>sis <strong>of</strong> quinuclidine. [51] Through utilization molecular hydrogen, heterogeneous<br />
hydrogenation catalysts Pt-C or Pd-C and Ti(OiPr) 4 <strong>for</strong> <strong>the</strong> reductive amination <strong>of</strong> a labile α-<br />
chiral quinuclidinone <strong>the</strong>y were successful in incorporation <strong>the</strong> amine without epimerization,<br />
a feat not previously accomplished (scheme 3.15).<br />
N<br />
Ph<br />
O<br />
Ph<br />
Ph NH 2<br />
Ti(OiPr) 4 /Pt-C/H 2 (4.2 bar)<br />
25 o C, 15h, 80% de<br />
N<br />
Ph<br />
NHCH 2 Ph<br />
Ph<br />
N<br />
Ph<br />
NHCH 2 Ph<br />
Ph<br />
Scheme 3.15. Reductive Amination <strong>of</strong> a Quinuclidinone<br />
Nugent group recently developed a two-step methodology relying on <strong>the</strong> asymmetric<br />
reductive amination <strong>of</strong> prochiral ketones with <strong>the</strong> chiral ammonia equivalent (R)- or (S)-αmethylbenzylamine<br />
<strong>for</strong> α-chiral primary amine syn<strong>the</strong>sis. They found that <strong>the</strong> use <strong>of</strong><br />
(Ti(O i Pr) 4 (1.2 equiv)) with ((R)- or (S)-α- methylbenzylamine (α-MBA) (1.1 equiv)) and<br />
heterogeneous catalyst (Ra-Ni, Pd-C, Pt-C) produced <strong>the</strong> secondary amine <strong>of</strong> different 2-<br />
alkanones, cyclic ketones, and aryl alkyl ketones in high yields and diastereoselectivities. The<br />
secondary amine is produced in a single step without <strong>the</strong> need <strong>for</strong> <strong>the</strong> tedious process <strong>of</strong><br />
imine isolation and purification. Simple acid base work is usually enough to purify <strong>the</strong><br />
65