Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
secondary amine product and any impurities like α-MBA (3-5%) can be removed easily<br />
through washing with NH 4 Cl. The primary amine is produced in high ee and yield through<br />
hydrogenolysis using Pd-C in MeOH and <strong>the</strong> ee can be fur<strong>the</strong>r enhanced through simple<br />
crystallization.<br />
Category 1: Raney-Ni<br />
Category 2: Pt-C<br />
HN<br />
Ph<br />
HN<br />
Ph<br />
HN<br />
Ph<br />
HN<br />
Ph<br />
76% yld,87% de<br />
1,2-dichloroethane<br />
94% yld, 74% de<br />
THF<br />
79% yld, 87% de<br />
hexane<br />
82% yld, 92% de<br />
ethanol<br />
Category 3: Pd-C<br />
Ph<br />
HN<br />
Ph<br />
Ph<br />
HN<br />
Ph<br />
NH 2<br />
H 2 N<br />
89% yld, 80% de<br />
methylenechloride<br />
92% yld, 94% de<br />
ethylacetate<br />
92% ee, overall yld 76%<br />
ethylacetate<br />
76% ee, overall yld 64%<br />
methyl-t-butyle<strong>the</strong>r<br />
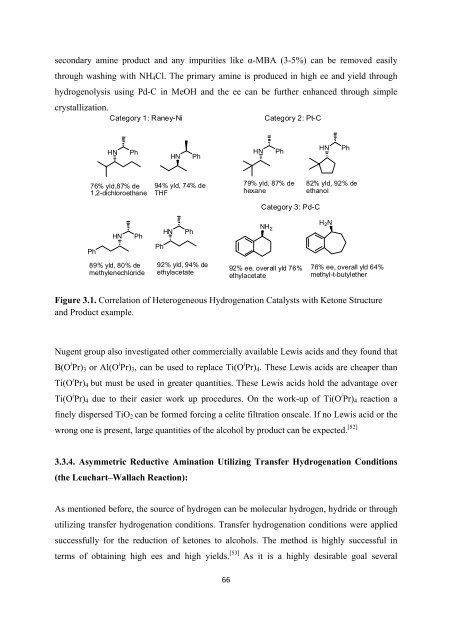
Figure 3.1. Correlation <strong>of</strong> Heterogeneous Hydrogenation Catalysts with Ketone Structure<br />
and Product example.<br />
Nugent group also investigated o<strong>the</strong>r commercially available Lewis acids and <strong>the</strong>y found that<br />
B(O i Pr) 3 or Al(O i Pr) 3 , can be used to replace Ti(O i Pr) 4 . These Lewis acids are cheaper than<br />
Ti(O i Pr) 4 but must be used in greater quantities. These Lewis acids hold <strong>the</strong> advantage over<br />
Ti(O i Pr) 4 due to <strong>the</strong>ir easier work up procedures. On <strong>the</strong> work-up <strong>of</strong> Ti(O i Pr) 4 reaction a<br />
finely dispersed TiO 2 can be <strong>for</strong>med <strong>for</strong>cing a celite filtration onscale. If no Lewis acid or <strong>the</strong><br />
wrong one is present, large quantities <strong>of</strong> <strong>the</strong> alcohol by product can be expected. [52]<br />
3.3.4. Asymmetric Reductive Amination Utilizing Transfer Hydrogenation Conditions<br />
(<strong>the</strong> Leuchart–Wallach Reaction):<br />
As mentioned be<strong>for</strong>e, <strong>the</strong> source <strong>of</strong> hydrogen can be molecular hydrogen, hydride or through<br />
utilizing transfer hydrogenation conditions. Transfer hydrogenation conditions were applied<br />
successfully <strong>for</strong> <strong>the</strong> reduction <strong>of</strong> ketones to alcohols. The method is highly successful in<br />
terms <strong>of</strong> obtaining high ees and high yields. [53] As it is a highly desirable goal several<br />
66