Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
You also want an ePaper? Increase the reach of your titles
YUMPU automatically turns print PDFs into web optimized ePapers that Google loves.
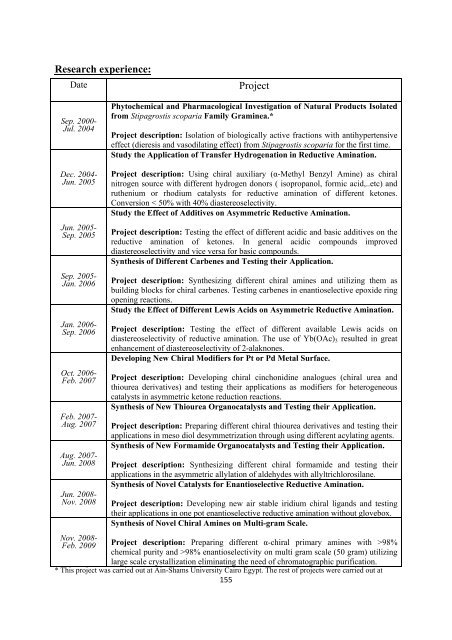
Research experience:<br />
Date<br />
Project<br />
Sep. 2000-<br />
Jul. 2004<br />
Phytochemical and Pharmacological Investigation <strong>of</strong> Natural Products Isolated<br />
from Stipagrostis scoparia Family Graminea.*<br />
Project description: Isolation <strong>of</strong> biologically active fractions with antihypertensive<br />
effect (dieresis and vasodilating effect) from Stipagrostis scoparia <strong>for</strong> <strong>the</strong> first time.<br />
Study <strong>the</strong> Application <strong>of</strong> Transfer Hydrogenation in Reductive Amination.<br />
Dec. 2004-<br />
Jun. 2005<br />
Jun. 2005-<br />
Sep. 2005<br />
Sep. 2005-<br />
Jan. 2006<br />
Jan. 2006-<br />
Sep. 2006<br />
Oct. 2006-<br />
Feb. 2007<br />
Feb. 2007-<br />
Aug. 2007<br />
Aug. 2007-<br />
Jun. 2008<br />
Jun. 2008-<br />
Nov. 2008<br />
Project description: Using chiral auxiliary (α-Methyl Benzyl Amine) as chiral<br />
nitrogen source with different hydrogen donors ( isopropanol, <strong>for</strong>mic acid,..etc) and<br />
ru<strong>the</strong>nium or rhodium catalysts <strong>for</strong> reductive amination <strong>of</strong> different ketones.<br />
Conversion < 50% with 40% diastereoselectivity.<br />
Study <strong>the</strong> Effect <strong>of</strong> Additives on Asymmetric Reductive Amination.<br />
Project description: Testing <strong>the</strong> effect <strong>of</strong> different acidic and basic additives on <strong>the</strong><br />
reductive amination <strong>of</strong> ketones. In general acidic compounds improved<br />
diastereoselectivity and vice versa <strong>for</strong> basic compounds.<br />
Syn<strong>the</strong>sis <strong>of</strong> Different Carbenes and Testing <strong>the</strong>ir Application.<br />
Project description: Syn<strong>the</strong>sizing different chiral amines and utilizing <strong>the</strong>m as<br />
building blocks <strong>for</strong> chiral carbenes. Testing carbenes in enantioselective epoxide ring<br />
opening reactions.<br />
Study <strong>the</strong> Effect <strong>of</strong> Different Lewis Acids on Asymmetric Reductive Amination.<br />
Project description: Testing <strong>the</strong> effect <strong>of</strong> different available Lewis acids on<br />
diastereoselectivity <strong>of</strong> reductive amination. The use <strong>of</strong> Yb(OAc) 3 resulted in great<br />
enhancement <strong>of</strong> diastereoselectivity <strong>of</strong> 2-alaknones.<br />
Developing New <strong>Chiral</strong> Modifiers <strong>for</strong> Pt or Pd Metal Surface.<br />
Project description: Developing chiral cinchonidine analogues (chiral urea and<br />
thiourea derivatives) and testing <strong>the</strong>ir applications as modifiers <strong>for</strong> heterogeneous<br />
catalysts in asymmetric ketone reduction reactions.<br />
Syn<strong>the</strong>sis <strong>of</strong> New Thiourea Organocatalysts and Testing <strong>the</strong>ir Application.<br />
Project description: Preparing different chiral thiourea derivatives and testing <strong>the</strong>ir<br />
applications in meso diol desymmetrization through using different acylating agents.<br />
Syn<strong>the</strong>sis <strong>of</strong> New Formamide Organocatalysts and Testing <strong>the</strong>ir Application.<br />
Project description: Syn<strong>the</strong>sizing different chiral <strong>for</strong>mamide and testing <strong>the</strong>ir<br />
applications in <strong>the</strong> asymmetric allylation <strong>of</strong> aldehydes with allyltrichlorosilane.<br />
Syn<strong>the</strong>sis <strong>of</strong> Novel Catalysts <strong>for</strong> Enantioselective Reductive Amination.<br />
Project description: Developing new air stable iridium chiral ligands and testing<br />
<strong>the</strong>ir applications in one pot enantioselective reductive amination without glovebox.<br />
Syn<strong>the</strong>sis <strong>of</strong> Novel <strong>Chiral</strong> <strong>Amines</strong> on Multi-gram Scale.<br />
Nov. 2008-<br />
Feb. 2009 Project description: Preparing different α-chiral primary amines with >98%<br />
chemical purity and >98% enantioselectivity on multi gram scale (50 gram) utilizing<br />
large scale crystallization eliminating <strong>the</strong> need <strong>of</strong> chromatographic purification.<br />
* This project was carried out at Ain-Shams University Cairo Egypt. The rest <strong>of</strong> projects were carried out at<br />
155