Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Improved Methodology for the Preparation of Chiral Amines
Create successful ePaper yourself
Turn your PDF publications into a flip-book with our unique Google optimized e-Paper software.
OH<br />
N<br />
O<br />
R<br />
1. 2LDA, LiCl<br />
2. R 1 X<br />
THF<br />
OH<br />
N<br />
O<br />
R 1<br />
R<br />
80-99% yield<br />
94-99% de<br />
OH<br />
N<br />
H 2 SO 4 dioxane<br />
O<br />
R<br />
N BH 3 Li<br />
R 1<br />
THF<br />
HO<br />
O<br />
R 1<br />
R<br />
HO<br />
O<br />
R<br />
H<br />
R 1<br />
87-97% yield<br />
95-97% ee<br />
R 1<br />
R<br />
75-92% yield<br />
90-98% ee<br />
80-88% yield<br />
88-99% ee<br />
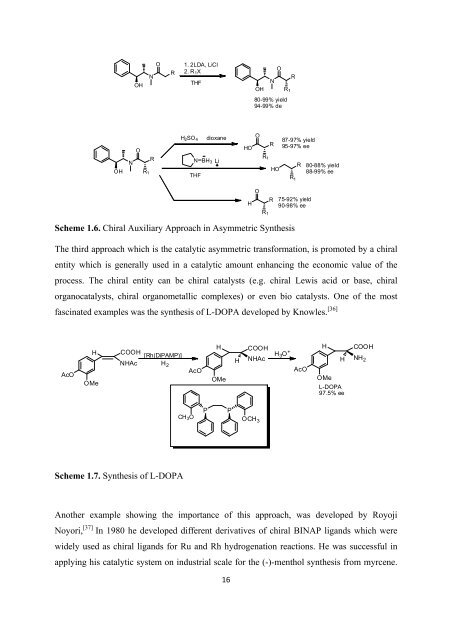
Scheme 1.6. <strong>Chiral</strong> Auxiliary Approach in Asymmetric Syn<strong>the</strong>sis<br />
The third approach which is <strong>the</strong> catalytic asymmetric trans<strong>for</strong>mation, is promoted by a chiral<br />
entity which is generally used in a catalytic amount enhancing <strong>the</strong> economic value <strong>of</strong> <strong>the</strong><br />
process. The chiral entity can be chiral catalysts (e.g. chiral Lewis acid or base, chiral<br />
organocatalysts, chiral organometallic complexes) or even bio catalysts. One <strong>of</strong> <strong>the</strong> most<br />
fascinated examples was <strong>the</strong> syn<strong>the</strong>sis <strong>of</strong> L-DOPA developed by Knowles. [36]<br />
AcO<br />
H<br />
OMe<br />
COOH [Rh(DiPAMP)]<br />
NHAc H 2<br />
AcO<br />
H<br />
OMe<br />
H<br />
COOH<br />
NHAc<br />
H 3 O +<br />
AcO<br />
H<br />
OMe<br />
H<br />
L-DOPA<br />
97.5% ee<br />
COOH<br />
NH 2<br />
CH 3 O<br />
P<br />
P<br />
OCH 3<br />
Scheme 1.7. Syn<strong>the</strong>sis <strong>of</strong> L-DOPA<br />
Ano<strong>the</strong>r example showing <strong>the</strong> importance <strong>of</strong> this approach, was developed by Royoji<br />
Noyori, [37] In 1980 he developed different derivatives <strong>of</strong> chiral BINAP ligands which were<br />
widely used as chiral ligands <strong>for</strong> Ru and Rh hydrogenation reactions. He was successful in<br />
applying his catalytic system on industrial scale <strong>for</strong> <strong>the</strong> (-)-menthol syn<strong>the</strong>sis from myrcene.<br />
16